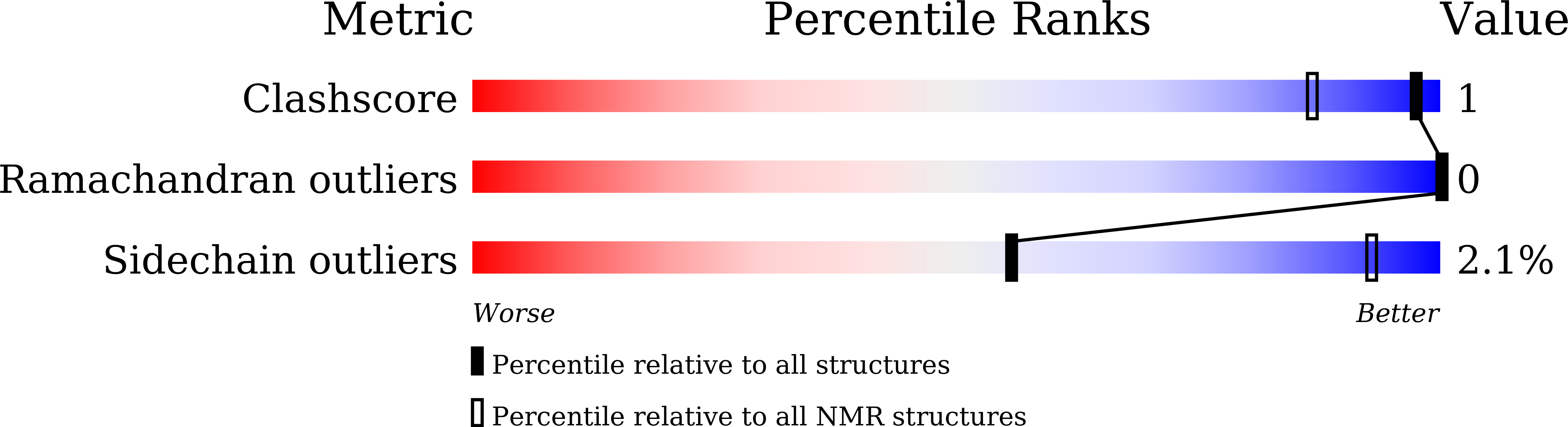Discovery of a cysteine-rich peptide with glycation modification from Achyranthes bidentata Blume.
He, M., Feng, Y., Wang, Y., Cheng, M., Zhang, X., Zhang, L.(2022) Fitoterapia 163: 105338-105338
- PubMed: 36270560
- DOI: https://doi.org/10.1016/j.fitote.2022.105338
- Primary Citation of Related Structures:
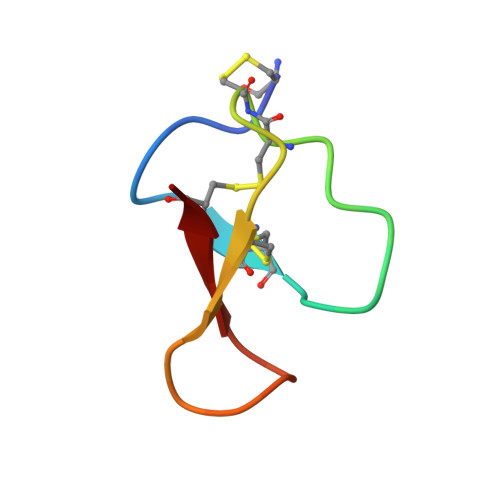
8HJC, 8HJD - PubMed Abstract:
Cysteine-rich peptides (CRPs) are stable molecules that contain multiple disulphide bonds. Various CRPs are found in plants and animals, representing potential compounds for drug development with diverse activities. Modification of CRPs, such as glycation, has attracted increased attention due to its special structural and functional properties. Hence, this study explored a CRP isolated from the Chinese herb Achyranthes bidentata Blume, which contains a glycation modification. Herein, a reverse phase high-performance liquid chromatography system with mobile phases was used to extract and purify the peptide. The eluted peptide was detected using high resolution mass spectrometry and structurally identified using high resolution mass spectrometry and nuclear magnetic resonance. The effect of the peptide on the viability of N-methyl-D-aspartic acid (NMDA)-induced HT22 cells was determined using a cell assay. Here, a new cysteine-rich glycation peptide, termed glycation-bidentatide (Gly-BTP), with three pairs of disulphide bonds and a glycation modification at the N-terminus linked to cysteine, was discovered. Cell bioactivity assay results suggested that Gly-BTP might be a potential therapeutic and provide a neuroprotective effect in NMDA-induced HT22 murine hippocampal neuronal cells. The discovery of Gly-BTP will promote the understanding of the role of CRPs in neuroprotection.
Organizational Affiliation:
CAS Key Laboratory of Separation Sciences of Analytical Chemistry, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 457 Zhongshan Road, Dalian 116023, China; University of Chinese Academy of Sciences, Yuquan Road 19, Beijing 100049, China.