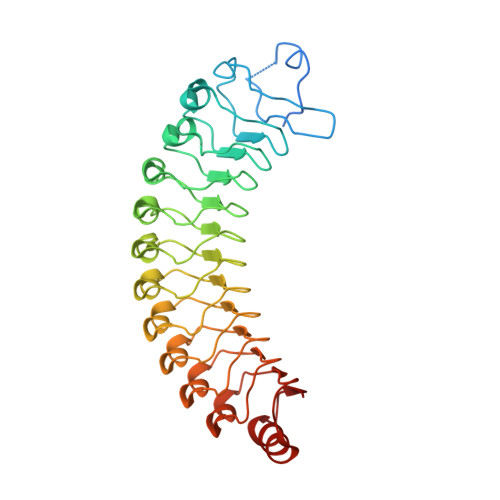
Crystal structure of Leptospira LSS_01692 reveals a dimeric structure and induces inflammatory responses through Toll-like receptor 2-dependent NF-kappa B and MAPK signal transduction pathways.
Hsu, S.H., Wu, C.T., Sun, Y.J., Chang, M.Y., Li, C., Ko, Y.C., Chou, L.F., Yang, C.W.(2023) FEBS J 290: 4513-4532
- PubMed: 37243454
- DOI: https://doi.org/10.1111/febs.16874
- Primary Citation of Related Structures:
7YJW - PubMed Abstract:
Leptospirosis is a commonly overlooked zoonotic disease that occurs in tropical and subtropical regions. Recent studies have divided the Leptospira spp. into three groups based on virulence, including pathogenic, intermediate, and saprophytic species. Pathogenic species express a protein family with leucine-rich repeat (LRR) domains, which are less expressed or absent in nonpathogenic species, highlighting the importance of this protein family in leptospirosis. However, the role of LRR domain proteins in the pathogenesis of leptospirosis is still unknown and requires further investigation. In this study, the 3D structure of LSS_01692 (rLRR38) was obtained using X-ray crystallography at a resolution of 3.2 Å. The results showed that rLRR38 forms a typical horseshoe structure with 11 α-helices and 11 β-sheets and an antiparallel dimeric structure. The interactions of rLRR38 with extracellular matrix and cell surface receptors were evaluated using ELISA and single-molecule atomic force microscopy. The results showed that rLRR38 interacted with fibronectin, collagen IV, and Toll-like receptor 2 (TLR2). Incubating HK2 cells with rLRR38 induced two downstream inflammation responses (IL-6 and MCP-1) in the TLR2 signal transduction pathway. The TLR2-TLR1 complex showed the most significant upregulation effects under rLRR38 treatment. Inhibitors also significantly inhibited nuclear factor κB and mitogen-activated protein kinases signals transduction under rLRR38 stimulation. In conclusion, rLRR38 was determined to be a novel LRR domain protein in 3D structure and demonstrated as a TLR2-binding protein that induces inflammatory responses. These structural and functional studies provide a deeper understanding of the pathogenesis of leptospirosis.
Organizational Affiliation:
Kidney Research Center, Chang Gung Memorial Hospital, Taoyuan, Taiwan.