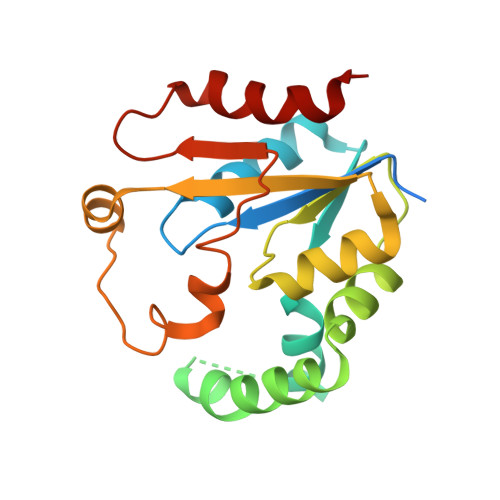
Structural insights into the substrate binding of phosphomevalonate kinase from the silkworm, Bombyx mori.
Zhang, H., Liu, J., Wang, H., Fang, H., Zhao, P., Xia, Q., Guo, P.(2022) Insect Biochem Mol Biol 150: 103849-103849
- PubMed: 36209956
- DOI: https://doi.org/10.1016/j.ibmb.2022.103849
- Primary Citation of Related Structures:
7XND - PubMed Abstract:
Phosphomevalonate kinase (PMK) is an important enzyme involved in the juvenile hormone (JH) biosynthesis pathway that catalyzes the phosphorylation of mevalonate 5-phosphate into mevalonate 5-diphosphate in the mevalonate pathway. Herein, we report the crystal structure of insect PMK from Bombyx mori (BmPMK) at a resolution of 1.60 Å. The overall structure of BmPMK adopts a compact α/β conformation with two parts: the core and lid regions. The interface between the core and lid regions forms a continuous and negatively charged groove to accommodate the substrates. Using computational simulation combined with site-directed mutagenesis and biochemical analysis, we define the binding mode of BmPMK with the cofactor and the substrate, which provides a structural basis for understanding the catalytic mechanism and the design of inhibitors of PMK. Moreover, BmPMK showed the optimal enzyme activity at pH 8.0, and the optimal temperature was 30 °C, using mevalonate 5-phosphate as the substrate. The expression profiles and kinetic analyses of BmPMK indicated that it plays critical role in the control of JH biosynthesis in silkworms. Collectively, these findings provide a better understanding of the structural and biochemical features of insect PMK.
Organizational Affiliation:
State Key Laboratory of Silkworm Genome Biology, Biological Science Research Center, Southwest University, Chongqing, 400716, China; Chongqing Key Laboratory of Sericultural Science, Chongqing Engineering and Technology Research Center for Novel Silk Materials, Southwest University, Chongqing, 400715, China.