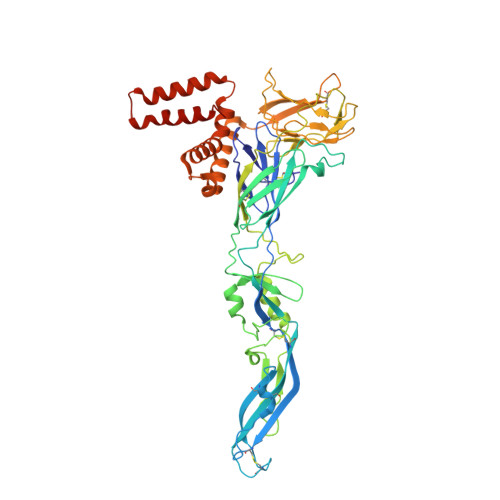
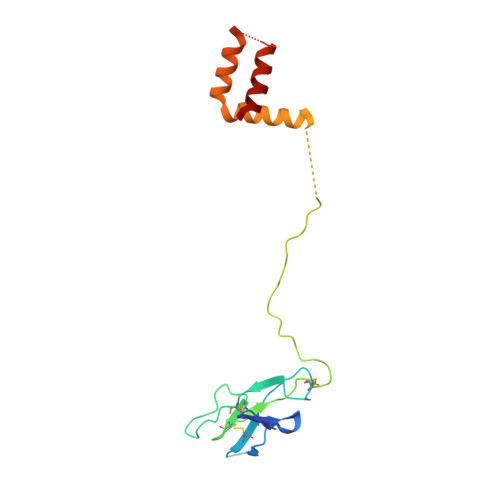
The structure of an infectious immature flavivirus redefines viral architecture and maturation.
Newton, N.D., Hardy, J.M., Modhiran, N., Hugo, L.E., Amarilla, A.A., Bibby, S., Venugopal, H., Harrison, J.J., Traves, R.J., Hall, R.A., Hobson-Peters, J., Coulibaly, F., Watterson, D.(2021) Sci Adv 7
- PubMed: 33990320
- DOI: https://doi.org/10.1126/sciadv.abe4507
- Primary Citation of Related Structures:
7L30 - PubMed Abstract:
Flaviviruses are the cause of severe human diseases transmitted by mosquitoes and ticks. These viruses use a potent fusion machinery to enter target cells that needs to be restrained during viral assembly and egress. A molecular chaperone, premembrane (prM) maintains the virus particles in an immature, fusion-incompetent state until they exit the cell. Taking advantage of an insect virus that produces particles that are both immature and infectious, we determined the structure of the first immature flavivirus with a complete spike by cryo-electron microscopy. Unexpectedly, the prM chaperone forms a supporting pillar that maintains the immature spike in an asymmetric and upright state, primed for large rearrangements upon acidification. The collapse of the spike along a path defined by the prM chaperone is required, and its inhibition by a multivalent immunoglobulin M blocks infection. The revised architecture and collapse model are likely to be conserved across flaviviruses.
Organizational Affiliation:
Australian Infectious Diseases Research Centre, School of Chemistry and Molecular Biosciences, The University of Queensland, Brisbane, QLD, Australia.