Alternative pathways utilize or circumvent putrescine for biosynthesis of putrescine-containing rhizoferrin.
Li, B., Deng, X., Kim, S.H., Buhrow, L., Tomchick, D.R., Phillips, M.A., Michael, A.J.(2020) J Biol Chem 296: 100146-100146
- PubMed: 33277357
- DOI: https://doi.org/10.1074/jbc.RA120.016738
- Primary Citation of Related Structures:
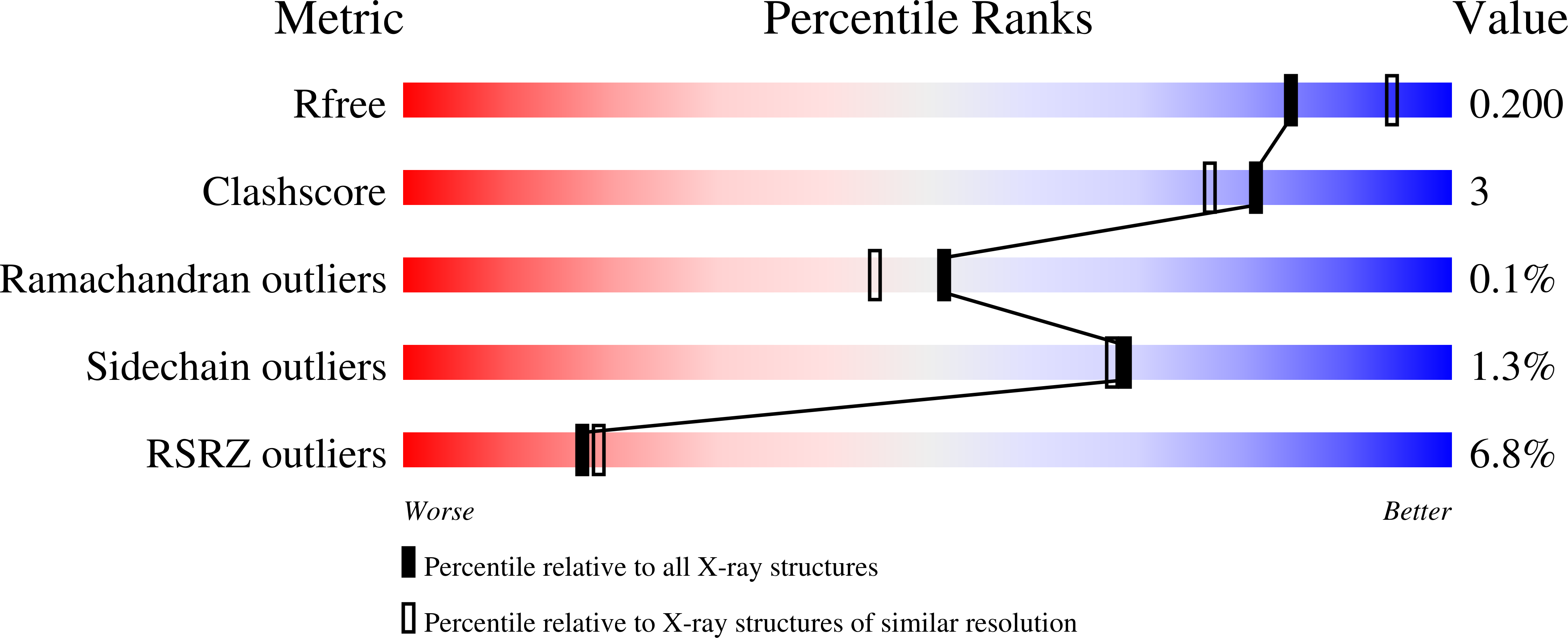
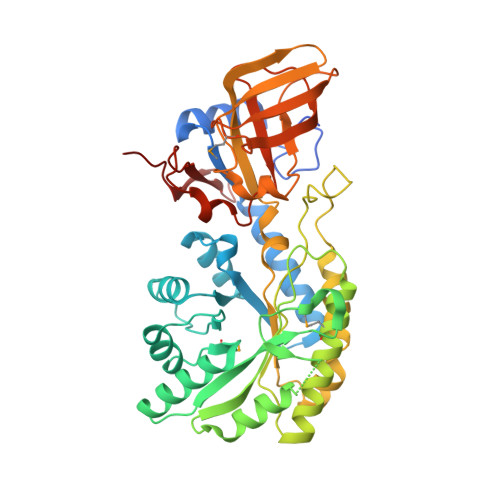
7KH2 - PubMed Abstract:
The siderophore rhizoferrin (N 1 ,N 4 -dicitrylputrescine) is produced in fungi and bacteria to scavenge iron. Putrescine-producing bacterium Ralstonia pickettii synthesizes rhizoferrin and encodes a single nonribosomal peptide synthetase-independent siderophore (NIS) synthetase. From biosynthetic logic, we hypothesized that this single enzyme is sufficient for rhizoferrin biosynthesis. We confirmed this by expression of R. pickettii NIS synthetase in Escherichia coli, resulting in rhizoferrin production. This was further confirmed in vitro using the recombinant NIS synthetase, synthesizing rhizoferrin from putrescine and citrate. Heterologous expression of homologous lbtA from Legionella pneumophila, required for rhizoferrin biosynthesis in that species, produced siderophore activity in E. coli. Rhizoferrin is also synthesized by Francisella tularensis and Francisella novicida, but unlike R. pickettii or L. pneumophila, Francisella species lack putrescine biosynthetic pathways because of genomic decay. Francisella encodes a NIS synthetase FslA/FigA and an ornithine decarboxylase homolog FslC/FigC, required for rhizoferrin biosynthesis. Ornithine decarboxylase produces putrescine from ornithine, but we show here in vitro that FigA synthesizes N-citrylornithine, and FigC is an N-citrylornithine decarboxylase that together synthesize rhizoferrin without using putrescine. We co-expressed F. novicida figA and figC in E. coli and produced rhizoferrin. A 2.1 Å X-ray crystal structure of the FigC N-citrylornithine decarboxylase reveals how the larger substrate is accommodated and how active site residues have changed to recognize N-citrylornithine. FigC belongs to a new subfamily of alanine racemase-fold PLP-dependent decarboxylases that are not involved in polyamine biosynthesis. These data reveal a natural product biosynthetic workaround that evolved to bypass a missing precursor and re-establish it in the final structure.
Organizational Affiliation:
Department of Biochemistry, UT Southwestern Medical Center, Dallas, Texas, USA.