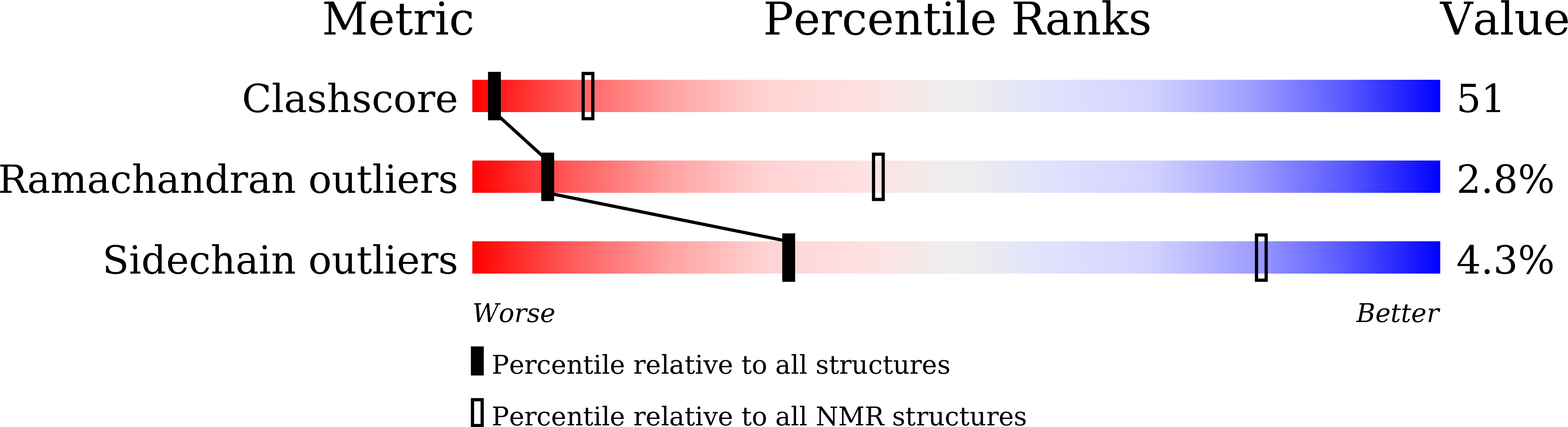Structural Insights into Methylated DNA Recognition by the Methyl-CpG Binding Domain of MBD6 from Arabidopsis thaliana .
Mahana, Y., Ohki, I., Walinda, E., Morimoto, D., Sugase, K., Shirakawa, M.(2022) ACS Omega 7: 3212-3221
- PubMed: 35128234
- DOI: https://doi.org/10.1021/acsomega.1c04917
- Primary Citation of Related Structures:
7D8K - PubMed Abstract:
Cytosine methylation is an epigenetic modification essential for formation of mature heterochromatin, gene silencing, and genomic stability. In plants, methylation occurs not only at cytosine bases in CpG but also in CpHpG and CpHpH contexts, where H denotes A, T, or C. Methyl-CpG binding domain (MBD) proteins, which recognize symmetrical methyl-CpG dinucleotides and act as gene repressors in mammalian cells, are also present in plant cells, although their structural and functional properties still remain poorly understood. To fill this gap, in this study, we determined the solution structure of the MBD domain of the MBD6 protein from Arabidopsis thaliana and investigated its binding properties to methylated DNA by binding assays and an in-depth NMR spectroscopic analysis. The AtMBD6 MBD domain folds into a canonical MBD structure in line with its binding specificity toward methyl-CpG and possesses a DNA binding interface similar to mammalian MBD domains. Intriguingly, however, the binding affinity of the AtMBD6 MBD domain toward methyl-CpG-containing DNA was found to be much lower than that of known mammalian MBD domains. The main difference arises from the absence of positively charged residues in AtMBD6 that supposedly interact with the DNA backbone as seen in mammalian MBD/methyl-CpG-containing DNA complexes. Taken together, we have established a structural basis for methyl-CpG recognition by AtMBD6 to develop a deeper understanding how MBD proteins work as mediators of epigenetic signals in plant cells.
Organizational Affiliation:
Department of Molecular Engineering, Kyoto University, Kyoto-Daigaku Katsura, Nishikyo-Ku, Kyoto 615-8510, Japan.