Crystal structure of the extracellular domain of the receptor-like kinase TMK3 from Arabidopsis thaliana.
Chen, H., Kong, Y., Chen, J., Li, L., Li, X., Yu, F., Ming, Z.(2020) Acta Crystallogr F Struct Biol Commun 76: 384-390
- PubMed: 32744250
- DOI: https://doi.org/10.1107/S2053230X20010122
- Primary Citation of Related Structures:
7BRC - PubMed Abstract:
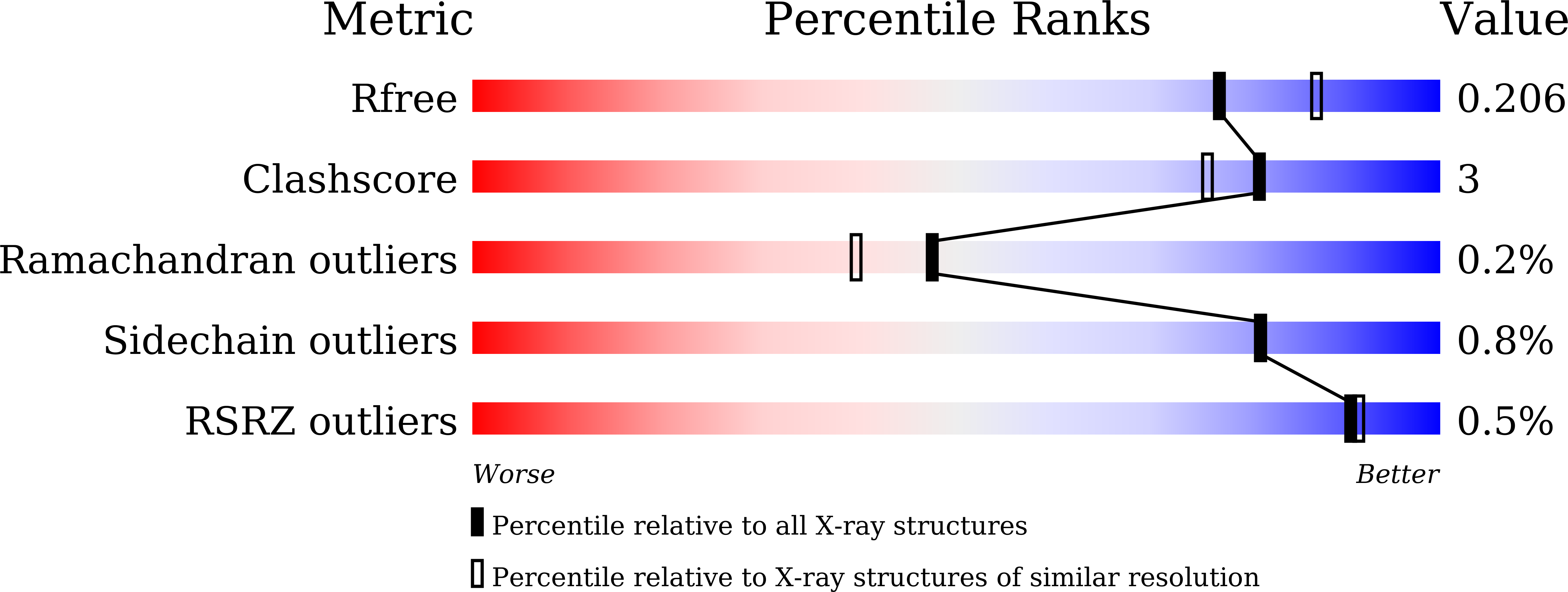
Transmembrane kinases (TMKs) are members of the plant receptor-like kinase (RLK) family. TMKs are characterized by an extracellular leucine-rich-repeat (LRR) domain, a single transmembrane region and a cytoplasmic kinase domain. TMKs have been shown to act as critical modulators of cell expansion and cell proliferation. Here, the crystal structure of the extracellular domain of TMK3 (TMK3-ECD) was determined to a resolution of 2.06 Å, with an R work of 17.69% and an R free of 20.58%. Similar to the extracellular domain of TMK1, the TMK3-ECD structure contains two solenoids with 13 LRRs and a non-LRR region (316-364) between the tenth and 11th LRRs. A comparison of TMK3-ECD with other LRR-RLKs that contain a non-LRR region indicates that the non-LRR region plays a critical role in structural integrity and may contribute to ligand interactions. The non-LRR region of TMK3-ECD is characterized by two disulfide bonds that may have critical biological implications.
Organizational Affiliation:
State Key Laboratory of Chemo/Biosensing and Chemometrics, College of Biology, Hunan Key Laboratory of Plant Functional Genomics and Developmental Regulation, Hunan University, Changsha 410082, People's Republic of China.