Crystal structure of the human METTL3-METTL14 complex bound to Compound 9 (ADO_AD_023)
Bedi, R.K., Dolbois, A., Caflisch, A.To be published.
Experimental Data Snapshot
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| N6-adenosine-methyltransferase catalytic subunit | 246 | Homo sapiens | Mutation(s): 0 Gene Names: METTL3, MTA70 EC: 2.1.1.348 | 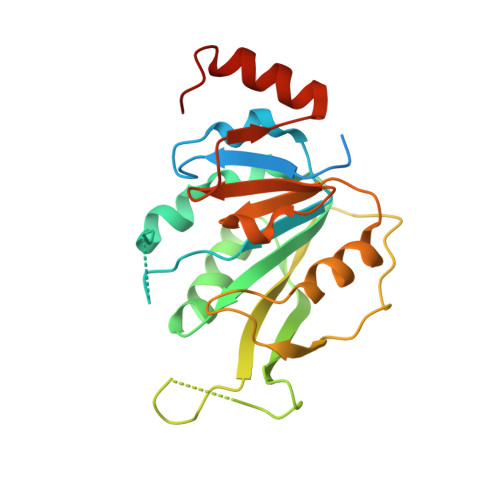 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q86U44 (Homo sapiens) Explore Q86U44 Go to UniProtKB: Q86U44 | |||||
PHAROS: Q86U44 GTEx: ENSG00000165819 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q86U44 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| N6-adenosine-methyltransferase non-catalytic subunit | 290 | Homo sapiens | Mutation(s): 0 Gene Names: METTL14, KIAA1627 | 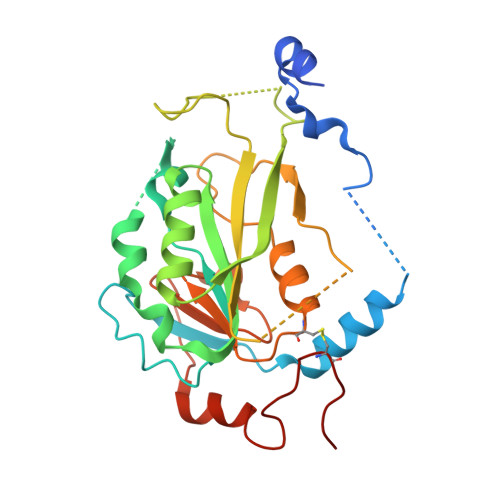 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q9HCE5 (Homo sapiens) Explore Q9HCE5 Go to UniProtKB: Q9HCE5 | |||||
PHAROS: Q9HCE5 GTEx: ENSG00000145388 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q9HCE5 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| UXT (Subject of Investigation/LOI) Query on UXT | C [auth A] | 4-[4-[(4,4-dimethylpiperidin-1-yl)methyl]phenyl]-9-[6-[(phenylmethyl)amino]pyrimidin-4-yl]-1,4,9-triazaspiro[5.5]undecan-2-one C33 H43 N7 O SMOMNPRYHLVUTG-UHFFFAOYSA-N |  | ||
| ACT Query on ACT | D [auth B] | ACETATE ION C2 H3 O2 QTBSBXVTEAMEQO-UHFFFAOYSA-M |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 64.07 | α = 90 |
| b = 64.07 | β = 90 |
| c = 225.08 | γ = 120 |
| Software Name | Purpose |
|---|---|
| XDS | data reduction |
| XSCALE | data scaling |
| PHASER | phasing |
| PHENIX | refinement |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Swiss National Science Foundation | Switzerland | 310030B_189363 |