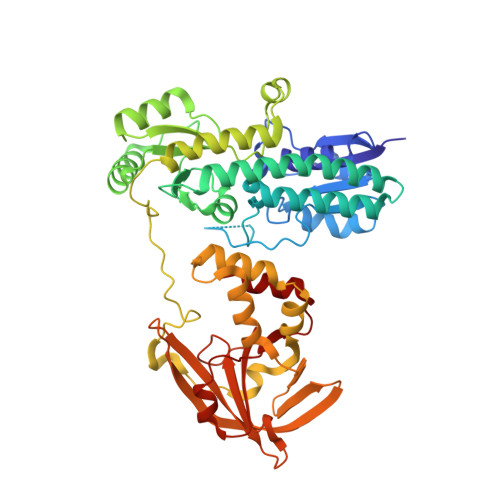
A Trap-Door Mechanism for Zinc Acquisition by Streptococcus pneumoniae AdcA.
Luo, Z., Morey, J.R., Deplazes, E., Motygullina, A., Tan, A., Ganio, K., Neville, S.L., Eleftheriadis, N., Isselstein, M., Pederick, V.G., Paton, J.C., Cordes, T., Harmer, J.R., Kobe, B., McDevitt, C.A.(2021) mBio 12
- PubMed: 33531394
- DOI: https://doi.org/10.1128/mBio.01958-20
- Primary Citation of Related Structures:
7JJ8, 7JJ9, 7JJA, 7JJB - PubMed Abstract:
Zinc is an essential element in all domains of life. Nonetheless, how prokaryotes achieve selective acquisition of zinc from the extracellular environment remains poorly understood. Here, we elucidate a novel mechanism for zinc-binding in AdcA, a solute-binding protein of Streptococcus pneumoniae Crystal structure analyses reveal the two-domain organization of the protein and show that only the N-terminal domain (AdcA N ) is necessary for zinc import. Zinc binding induces only minor changes in the global protein conformation of AdcA and stabilizes a highly mobile loop within the AdcA N domain. This loop region, which is conserved in zinc-specific solute-binding proteins, facilitates closure of the AdcA N binding site and is crucial for zinc acquisition. Collectively, these findings elucidate the structural and functional basis of selective zinc uptake in prokaryotes. IMPORTANCE Zinc is an essential nutrient for the virulence of bacterial pathogens such as Streptococcus pneumoniae Many Gram-positive bacteria use a two-domain lipoprotein for zinc acquisition, but how this class of metal-recruiting proteins acquire zinc and interact with the uptake machinery has remained poorly defined. We report the first structure of a two-domain lipoprotein, AdcA from S. pneumoniae , and use computational, spectroscopic, and microbiological approaches to provide new insights into the functional basis of zinc recruitment. Our findings reveal that AdcA employs a novel mechanism for zinc binding that we have termed the "trap-door" mechanism, and we show how the static metal-binding site of the protein, which confers its selectivity for zinc ions, is combined with a dynamic surface element to facilitate zinc recruitment and import into the bacterium. Together, these findings expand our understanding of how bacteria acquire zinc from the environment and provide a foundation for inhibiting this process, through antimicrobial targeting of the dynamic structural elements to block bacterial zinc scavenging.
Organizational Affiliation:
School of Chemistry and Molecular Biosciences, University of Queensland, Brisbane, Queensland, Australia.