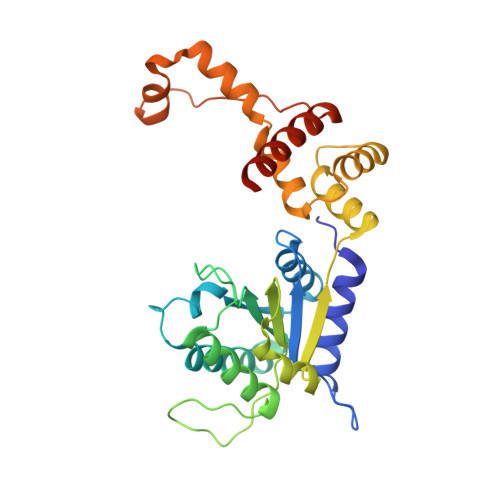
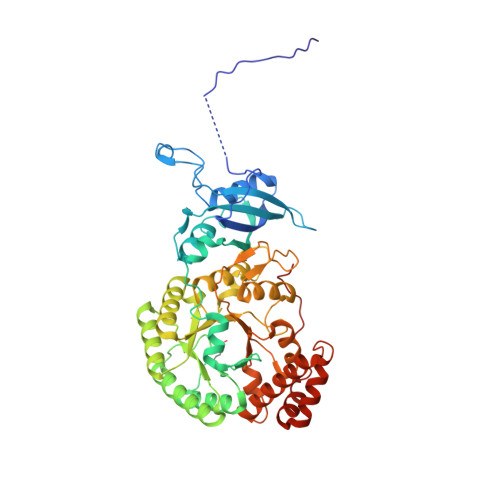
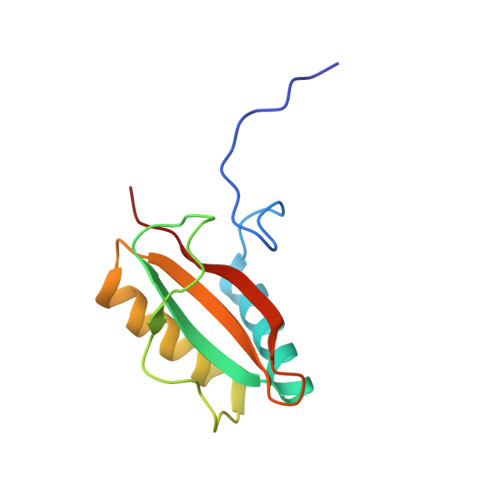
Dual Functions of a Rubisco Activase in Metabolic Repair and Recruitment to Carboxysomes.
Flecken, M., Wang, H., Popilka, L., Hartl, F.U., Bracher, A., Hayer-Hartl, M.(2020) Cell 183: 457-473.e20
- PubMed: 32979320
- DOI: https://doi.org/10.1016/j.cell.2020.09.010
- Primary Citation of Related Structures:
6HAS, 6Z1D, 6Z1E, 6Z1F, 6Z1G - PubMed Abstract:
Rubisco, the key enzyme of CO 2 fixation in photosynthesis, is prone to inactivation by inhibitory sugar phosphates. Inhibited Rubisco undergoes conformational repair by the hexameric AAA+ chaperone Rubisco activase (Rca) in a process that is not well understood. Here, we performed a structural and mechanistic analysis of cyanobacterial Rca, a close homolog of plant Rca. In the Rca:Rubisco complex, Rca is positioned over the Rubisco catalytic site under repair and pulls the N-terminal tail of the large Rubisco subunit (RbcL) into the hexamer pore. Simultaneous displacement of the C terminus of the adjacent RbcL opens the catalytic site for inhibitor release. An alternative interaction of Rca with Rubisco is mediated by C-terminal domains that resemble the small Rubisco subunit. These domains, together with the N-terminal AAA+ hexamer, ensure that Rca is packaged with Rubisco into carboxysomes. The cyanobacterial Rca is a dual-purpose protein with functions in Rubisco repair and carboxysome organization.
Organizational Affiliation:
Department of Cellular Biochemistry, Max Planck Institute of Biochemistry, Am Klopferspitz 18, 82152 Martinsried, Germany.