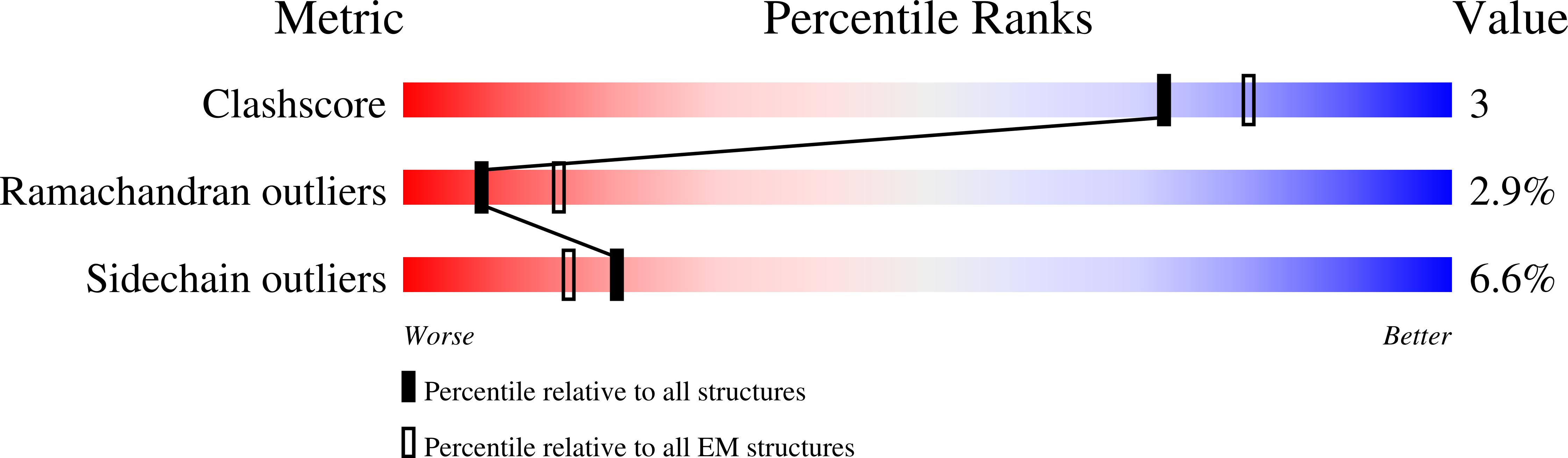Functional analysis and cryo-electron microscopy of Campylobacter jejuni serine protease HtrA.
Zarzecka, U., Grinzato, A., Kandiah, E., Cysewski, D., Berto, P., Skorko-Glonek, J., Zanotti, G., Backert, S.(2020) Gut Microbes 12: 1-16
- PubMed: 32960677
- DOI: https://doi.org/10.1080/19490976.2020.1810532
- Primary Citation of Related Structures:
6Z05 - PubMed Abstract:
Campylobacter jejuni is a predominant zoonotic pathogen causing gastroenteritis and other diseases in humans. An important bacterial virulence factor is the secreted serine protease HtrA (HtrA Cj ), which targets tight and adherens junctional proteins in the gut epithelium. Here we have investigated the function and structure of HtrA Cj using biochemical assays and cryo-electron microscopy. Mass spectrometry analysis identified differences and similarities in the cleavage site specificity for HtrA Cj by comparison to the HtrA counterparts from Helicobacter pylori and Escherichia coli . We defined the architecture of HtrA Cj at 5.8 Å resolution as a dodecamer, built of four trimers. The contacts between the trimers are quite loose, a fact that explains the flexibility and mobility of the dodecameric assembly. This flexibility has also been studied through molecular dynamics simulation, which revealed opening of the dodecamer to expose the proteolytically active site of the protease. Moreover, we examined the rearrangements at the level of oligomerization in the presence or absence of substrate using size exclusion chromatography, which revealed hexamers, dodecamers and larger oligomeric forms, as well as remarkable stability of higher oligomeric forms (> 12-mers) compared to previously tested homologs from other bacteria. Extremely dynamic decay of the higher oligomeric forms into lower forms was observed after full cleavage of the substrate by the proteolytically active variant of HtrA Cj . Together, this is the first report on the in-depth functional and structural analysis of HtrA Cj , which may allow the construction of therapeutically relevant HtrA Cj inhibitors in the near future.
Organizational Affiliation:
Division of Microbiology, Department of Biology, Friedrich-Alexander-University Erlangen-Nuremberg , Erlangen, Germany.