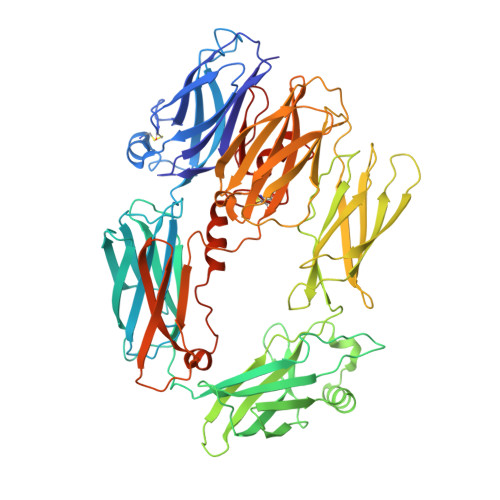
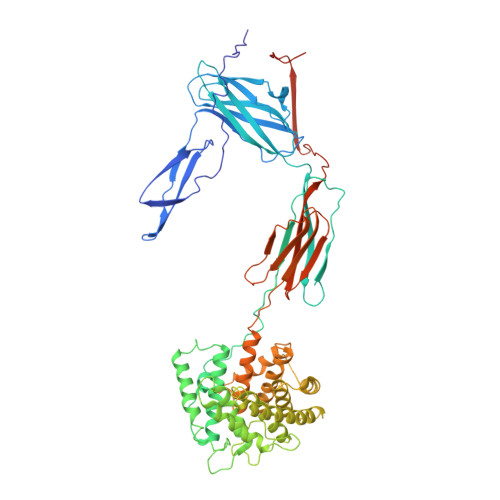
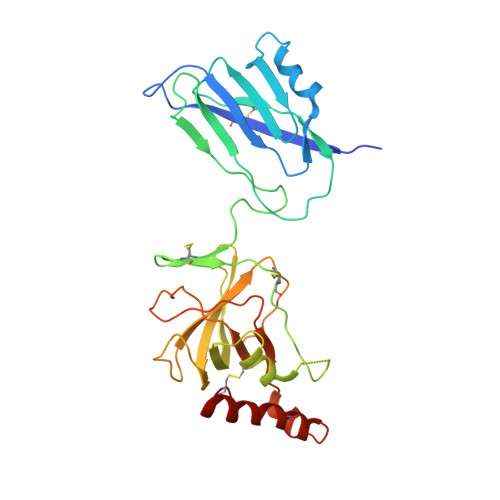
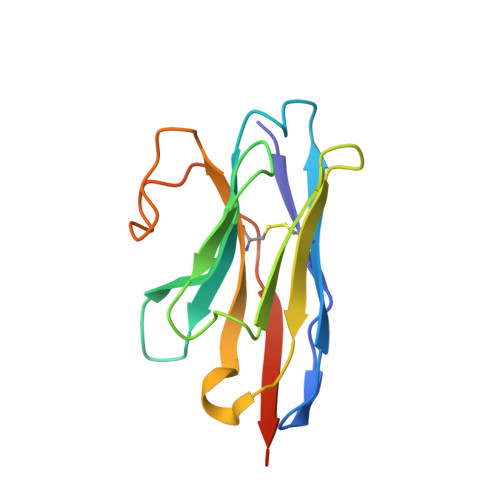
An Ultrahigh-Affinity Complement C4b-Specific Nanobody Inhibits In Vivo Assembly of the Classical Pathway Proconvertase.
Zarantonello, A., Presumey, J., Simoni, L., Yalcin, E., Fox, R., Hansen, A., Olesen, H.G., Thiel, S., Johnson, M.B., Stevens, B., Laursen, N.S., Carroll, M.C., Andersen, G.R.(2020) J Immunol 205: 1678-1694
- PubMed: 32769120
- DOI: https://doi.org/10.4049/jimmunol.2000528
- Primary Citation of Related Structures:
6YSQ - PubMed Abstract:
The classical and lectin pathways of the complement system are important for the elimination of pathogens and apoptotic cells and stimulation of the adaptive immune system. Upon activation of these pathways, complement component C4 is proteolytically cleaved, and the major product C4b is deposited on the activator, enabling assembly of a C3 convertase and downstream alternative pathway amplification. Although excessive activation of the lectin and classical pathways contributes to multiple autoimmune and inflammatory diseases and overexpression of a C4 isoform has recently been linked to schizophrenia, a C4 inhibitor and structural characterization of the convertase formed by C4b is lacking. In this study, we present the nanobody hC4Nb8 that binds with picomolar affinity to human C4b and potently inhibits in vitro complement C3 deposition through the classical and lectin pathways in human serum and in mouse serum. The crystal structure of the C4b:hC4Nb8 complex and a three-dimensional reconstruction of the C4bC2 proconvertase obtained by electron microscopy together rationalize how hC4Nb8 prevents proconvertase assembly through recognition of a neoepitope exposed in C4b and reveals a unique C2 conformation compared with the alternative pathway proconvertase. On human induced pluripotent stem cell-derived neurons, the nanobody prevents C3 deposition through the classical pathway. Furthermore, hC4Nb8 inhibits the classical pathway-mediated immune complex delivery to follicular dendritic cells in vivo. The hC4Nb8 represents a novel ultrahigh-affinity inhibitor of the classical and lectin pathways of the complement cascade under both in vitro and in vivo conditions.
Organizational Affiliation:
Department of Molecular Biology and Genetics, Aarhus University, DK8000 Aarhus, Denmark.