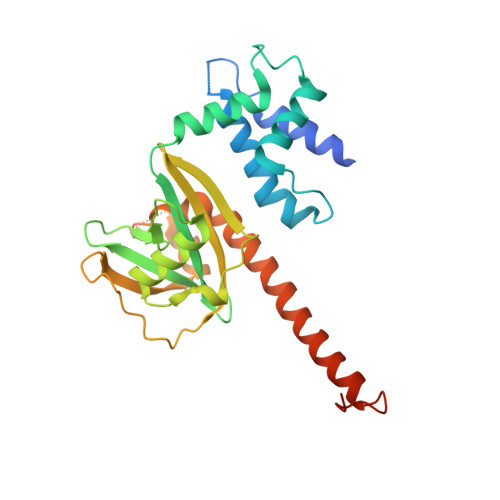
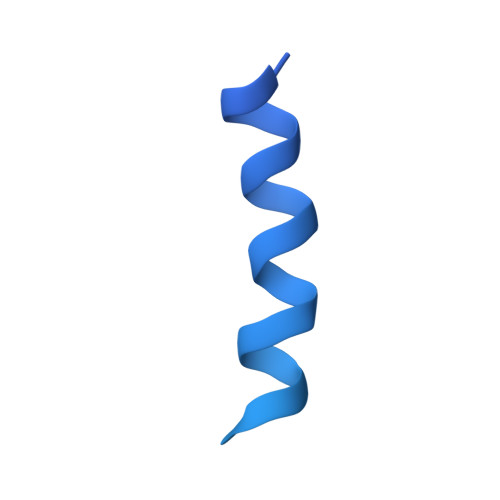
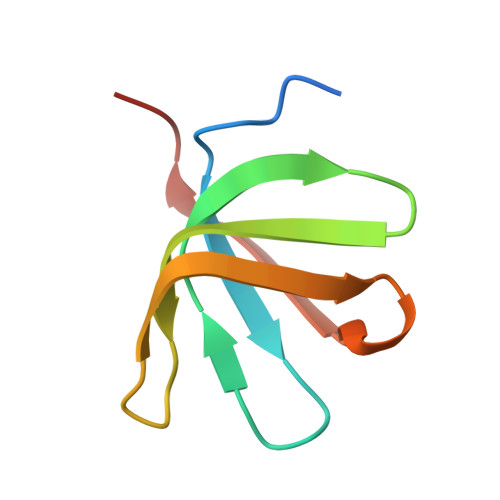
Pby1 is a direct partner of the Dcp2 decapping enzyme.
Charenton, C., Gaudon-Plesse, C., Back, R., Ulryck, N., Cosson, L., Seraphin, B., Graille, M.(2020) Nucleic Acids Res 48: 6353-6366
- PubMed: 32396195
- DOI: https://doi.org/10.1093/nar/gkaa337
- Primary Citation of Related Structures:
6Y3P, 6Y3Z - PubMed Abstract:
Most eukaryotic mRNAs harbor a characteristic 5' m7GpppN cap that promotes pre-mRNA splicing, mRNA nucleocytoplasmic transport and translation while also protecting mRNAs from exonucleolytic attacks. mRNA caps are eliminated by Dcp2 during mRNA decay, allowing 5'-3' exonucleases to degrade mRNA bodies. However, the Dcp2 decapping enzyme is poorly active on its own and requires binding to stable or transient protein partners to sever the cap of target mRNAs. Here, we analyse the role of one of these partners, the yeast Pby1 factor, which is known to co-localize into P-bodies together with decapping factors. We report that Pby1 uses its C-terminal domain to directly bind to the decapping enzyme. We solved the structure of this Pby1 domain alone and bound to the Dcp1-Dcp2-Edc3 decapping complex. Structure-based mutant analyses reveal that Pby1 binding to the decapping enzyme is required for its recruitment into P-bodies. Moreover, Pby1 binding to the decapping enzyme stimulates growth in conditions in which decapping activation is compromised. Our results point towards a direct connection of Pby1 with decapping and P-body formation, both stemming from its interaction with the Dcp1-Dcp2 holoenzyme.
Organizational Affiliation:
Laboratoire de Biologie Structurale de la Cellule (BIOC), CNRS, Ecole polytechnique, IP Paris, 91128 Palaiseau, France.