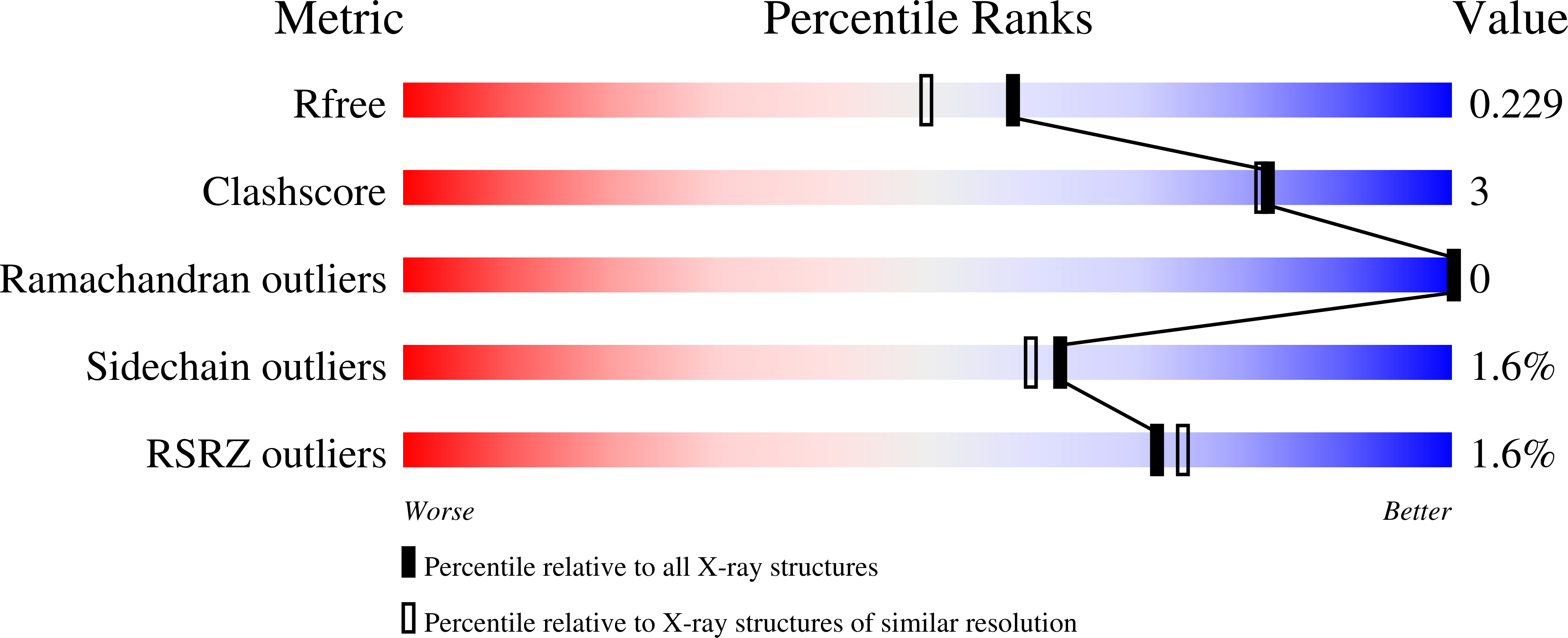
Megabodies expand the nanobody toolkit for protein structure determination by single-particle cryo-EM.
Uchanski, T., Masiulis, S., Fischer, B., Kalichuk, V., Lopez-Sanchez, U., Zarkadas, E., Weckener, M., Sente, A., Ward, P., Wohlkonig, A., Zogg, T., Remaut, H., Naismith, J.H., Nury, H., Vranken, W., Aricescu, A.R., Pardon, E., Steyaert, J.(2021) Nat Methods 18: 60-68
- PubMed: 33408403
- DOI: https://doi.org/10.1038/s41592-020-01001-6
- Primary Citation of Related Structures:
6QFA, 6XUX, 6XV8, 6XVI - PubMed Abstract:
Nanobodies are popular and versatile tools for structural biology. They have a compact single immunoglobulin domain organization, bind target proteins with high affinities while reducing their conformational heterogeneity and stabilize multi-protein complexes. Here we demonstrate that engineered nanobodies can also help overcome two major obstacles that limit the resolution of single-particle cryo-electron microscopy reconstructions: particle size and preferential orientation at the water-air interfaces. We have developed and characterized constructs, termed megabodies, by grafting nanobodies onto selected protein scaffolds to increase their molecular weight while retaining the full antigen-binding specificity and affinity. We show that the megabody design principles are applicable to different scaffold proteins and recognition domains of compatible geometries and are amenable for efficient selection from yeast display libraries. Moreover, we demonstrate that megabodies can be used to obtain three-dimensional reconstructions for membrane proteins that suffer from severe preferential orientation or are otherwise too small to allow accurate particle alignment.
Organizational Affiliation:
Structural Biology Brussels, Vrije Universiteit Brussel, VUB, Brussels, Belgium.