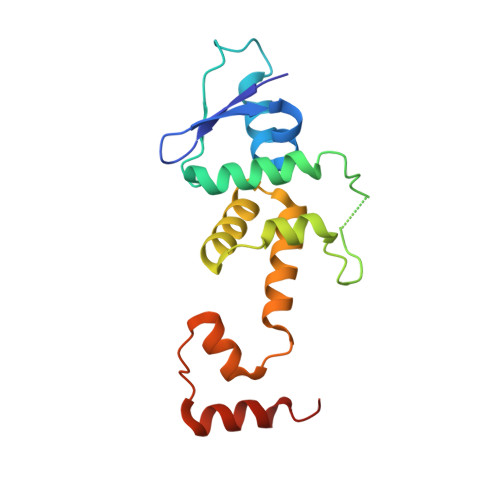
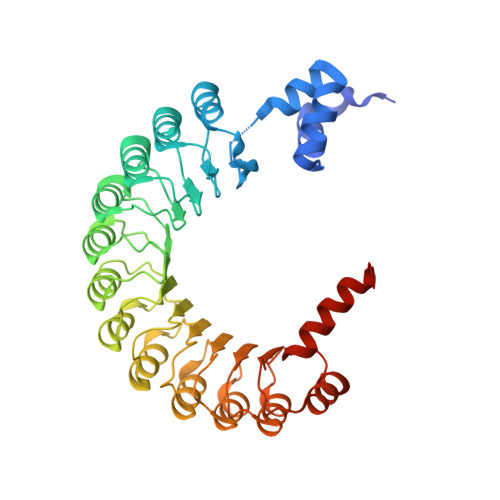
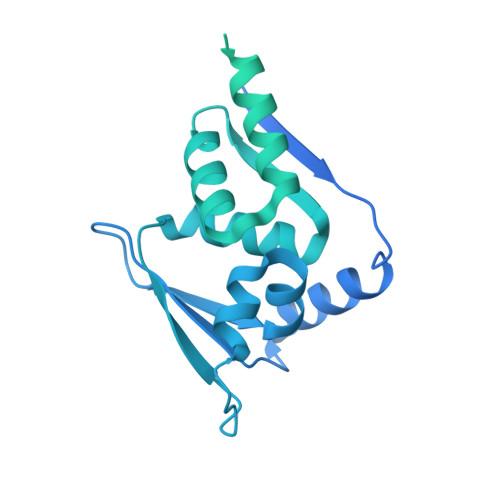
Structural basis for dimerization quality control.
Mena, E.L., Jevtic, P., Greber, B.J., Gee, C.L., Lew, B.G., Akopian, D., Nogales, E., Kuriyan, J., Rape, M.(2020) Nature 586: 452-456
- PubMed: 32814905
- DOI: https://doi.org/10.1038/s41586-020-2636-7
- Primary Citation of Related Structures:
6W66, 6W67, 6W68, 6W69, 6WCQ - PubMed Abstract:
Most quality control pathways target misfolded proteins to prevent toxic aggregation and neurodegeneration 1 . Dimerization quality control further improves proteostasis by eliminating complexes of aberrant composition 2 , but how it detects incorrect subunits remains unknown. Here we provide structural insight into target selection by SCF-FBXL17, a dimerization-quality-control E3 ligase that ubiquitylates and helps to degrade inactive heterodimers of BTB proteins while sparing functional homodimers. We find that SCF-FBXL17 disrupts aberrant BTB dimers that fail to stabilize an intermolecular β-sheet around a highly divergent β-strand of the BTB domain. Complex dissociation allows SCF-FBXL17 to wrap around a single BTB domain, resulting in robust ubiquitylation. SCF-FBXL17 therefore probes both shape and complementarity of BTB domains, a mechanism that is well suited to establish quality control of complex composition for recurrent interaction modules.
Organizational Affiliation:
Department of Molecular and Cell Biology, University of California at Berkeley, Berkeley, CA, USA.