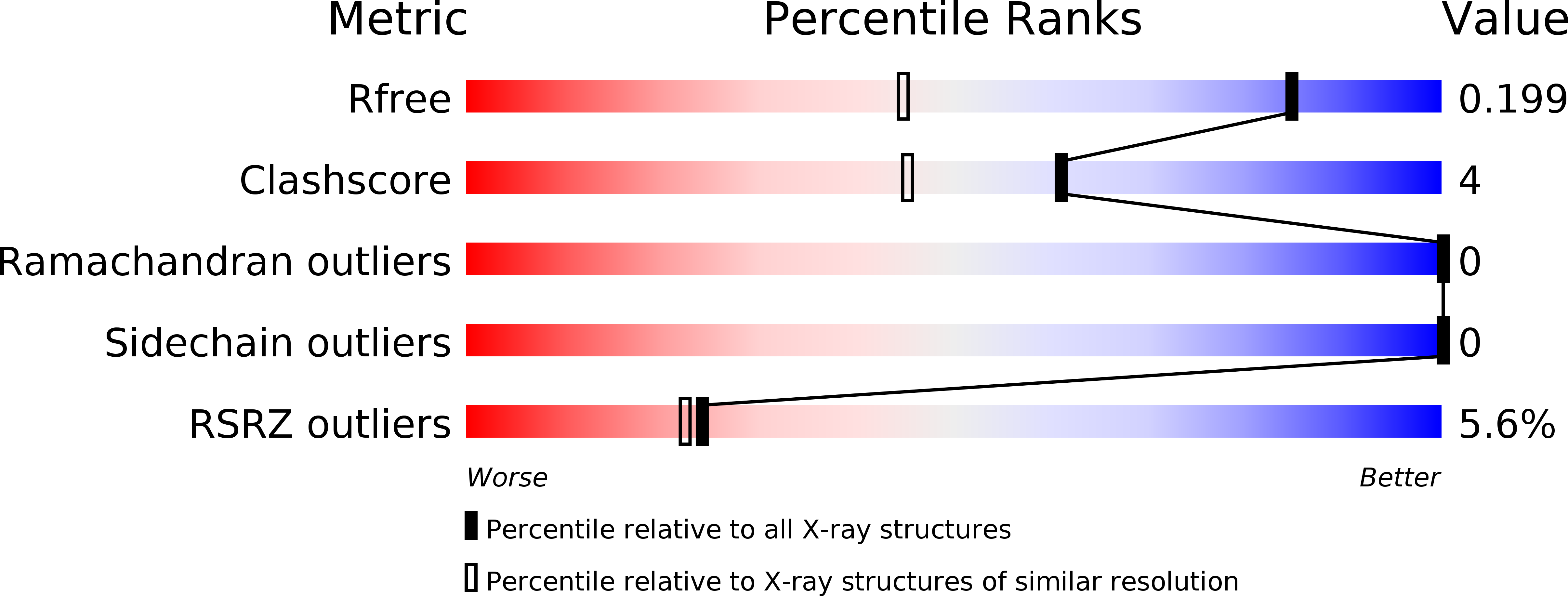
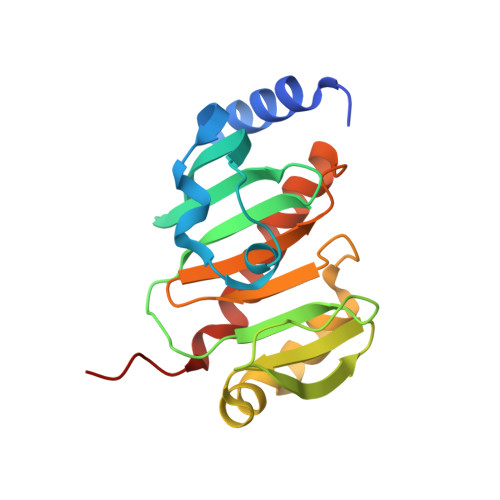
The crystal structure of the mycobacterial trehalose monomycolate transport factor A, TtfA, reveals an atypical fold.
Ung, K.L., Alsarraf, H.M.A.B., Kremer, L., Blaise, M.(2020) Proteins 88: 809-815
- PubMed: 31833106
- DOI: https://doi.org/10.1002/prot.25863
- Primary Citation of Related Structures:
6T84 - PubMed Abstract:
Trehalose monomycolate (TMM) represents an essential element of the mycobacterial envelope. While synthesized in the cytoplasm, TMM is transported across the inner membrane by MmpL3 but, little is known regarding the MmpL3 partners involved in this process. Recently, the TMM transport factor A (TtfA) was found to form a complex with MmpL3 and to participate in TMM transport, although its biological role remains to be established. Herein, we report the crystal structure of the Mycobacterium smegmatis TtfA core domain. The phylogenetic distribution of TtfA homologues in non-mycolate containing bacteria suggests that TtfA may exert additional functions.
Organizational Affiliation:
Institut de Recherche en Infectiologie de Montpellier (IRIM), Université de Montpellier, CNRS UMR 9004, Montpellier, France.