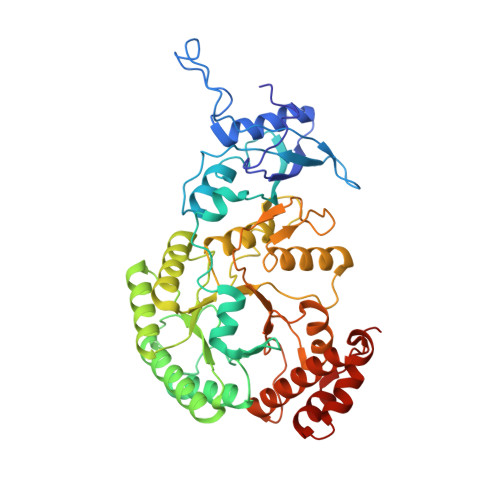
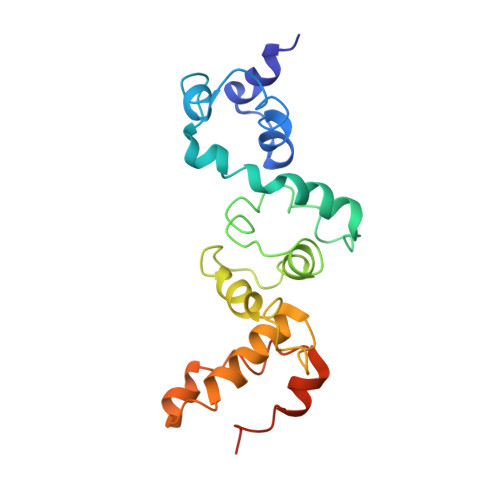
Rubisco accumulation factor 1 (Raf1) plays essential roles in mediating Rubisco assembly and carboxysome biogenesis.
Huang, F., Kong, W.W., Sun, Y., Chen, T., Dykes, G.F., Jiang, Y.L., Liu, L.N.(2020) Proc Natl Acad Sci U S A 117: 17418-17428
- PubMed: 32636267
- DOI: https://doi.org/10.1073/pnas.2007990117
- Primary Citation of Related Structures:
6SMH - PubMed Abstract:
Carboxysomes are membrane-free organelles for carbon assimilation in cyanobacteria. The carboxysome consists of a proteinaceous shell that structurally resembles virus capsids and internal enzymes including ribulose 1,5-bisphosphate carboxylase/oxygenase (Rubisco), the primary carbon-fixing enzyme in photosynthesis. The formation of carboxysomes requires hierarchical self-assembly of thousands of protein subunits, initiated from Rubisco assembly and packaging to shell encapsulation. Here we study the role of Rubisco assembly factor 1 (Raf1) in Rubisco assembly and carboxysome formation in a model cyanobacterium, Synechococcus elongatus PCC7942 (Syn7942). Cryo-electron microscopy reveals that Raf1 facilitates Rubisco assembly by mediating RbcL dimer formation and dimer-dimer interactions. Syn7942 cells lacking Raf1 are unable to form canonical intact carboxysomes but generate a large number of intermediate assemblies comprising Rubisco, CcaA, CcmM, and CcmN without shell encapsulation and a low abundance of carboxysome-like structures with reduced dimensions and irregular shell shapes and internal organization. As a consequence, the Raf1-depleted cells exhibit reduced Rubisco content, CO 2 -fixing activity, and cell growth. Our results provide mechanistic insight into the chaperone-assisted Rubisco assembly and biogenesis of carboxysomes. Advanced understanding of the biogenesis and stepwise formation process of the biogeochemically important organelle may inform strategies for heterologous engineering of functional CO 2 -fixing modules to improve photosynthesis.
Organizational Affiliation:
Institute of Integrative Biology, University of Liverpool, L69 7ZB Liverpool, United Kingdom.