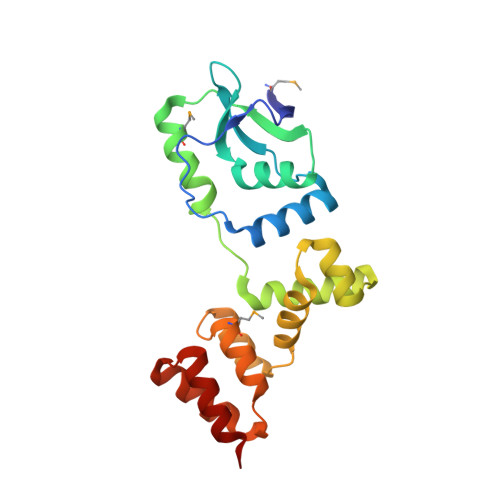
Self-organization ofparScentromeres by the ParB CTP hydrolase.
Soh, Y.M., Davidson, I.F., Zamuner, S., Basquin, J., Bock, F.P., Taschner, M., Veening, J.W., De Los Rios, P., Peters, J.M., Gruber, S.(2019) Science 366: 1129-1133
- PubMed: 31649139
- DOI: https://doi.org/10.1126/science.aay3965
- Primary Citation of Related Structures:
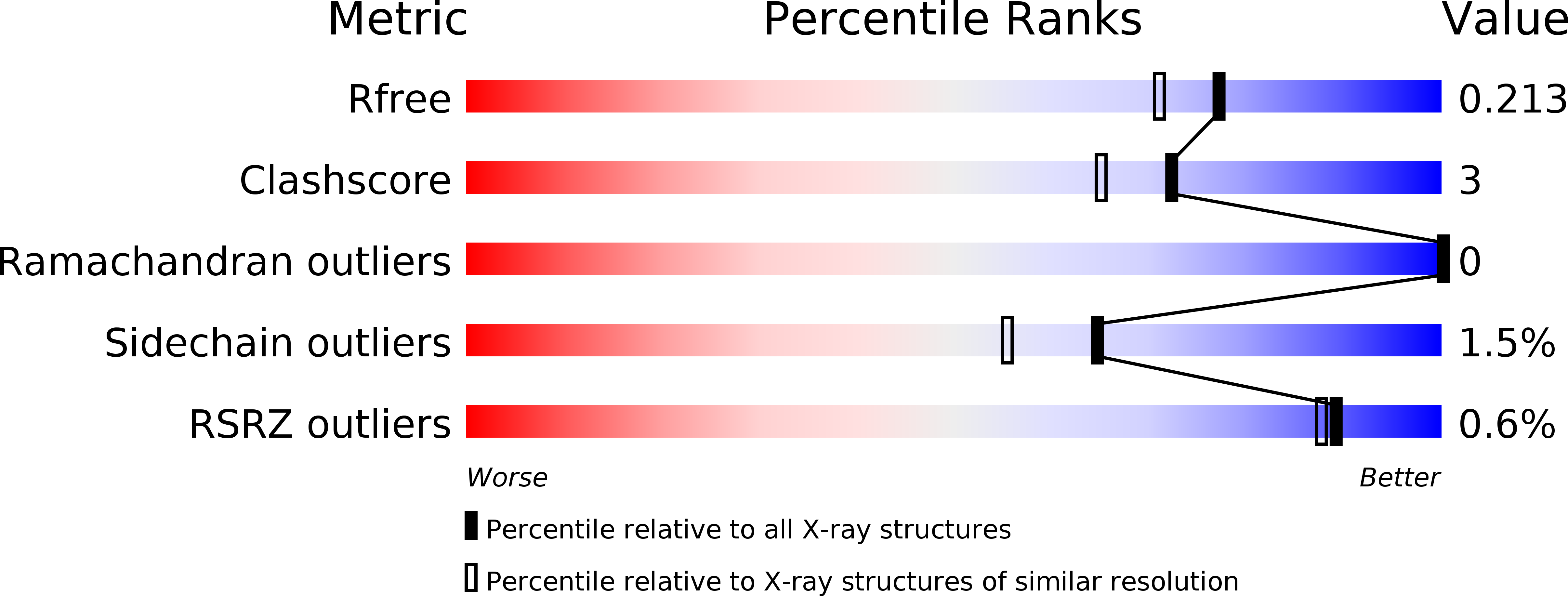
6SDK - PubMed Abstract:
ParABS systems facilitate chromosome segregation and plasmid partitioning in bacteria and archaea. ParB protein binds centromeric parS DNA sequences and spreads to flanking DNA. We show that ParB is an enzyme that hydrolyzes cytidine triphosphate (CTP) to cytidine diphosphate (CDP). parS DNA stimulates cooperative CTP binding by ParB and CTP hydrolysis. A nucleotide cocrystal structure elucidates the catalytic center of the dimerization-dependent ParB CTPase. Single-molecule imaging and biochemical assays recapitulate features of ParB spreading from parS in the presence but not absence of CTP. These findings suggest that centromeres assemble by self-loading of ParB DNA sliding clamps at parS ParB CTPase is not related to known nucleotide hydrolases and might be a promising target for developing new classes of antibiotics.
Organizational Affiliation:
Department of Fundamental Microbiology (DMF), Faculty of Biology and Medicine (FBM), University of Lausanne (UNIL), Lausanne, Switzerland.