YtrASa, a GntR-Family Transcription Factor, Represses Two Genetic Loci Encoding Membrane Proteins inSulfolobus acidocaldarius.
Lemmens, L., Tilleman, L., De Koning, E., Valegard, K., Lindas, A.C., Van Nieuwerburgh, F., Maes, D., Peeters, E.(2019) Front Microbiol 10: 2084-2084
- PubMed: 31552000
- DOI: https://doi.org/10.3389/fmicb.2019.02084
- Primary Citation of Related Structures:
6SBS - PubMed Abstract:
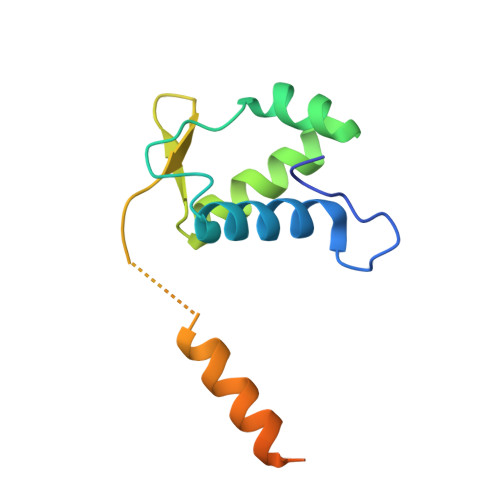
In bacteria, the GntR family is a widespread family of transcription factors responsible for the regulation of a myriad of biological processes. In contrast, despite their occurrence in archaea only a little information is available on the function of GntR-like transcription factors in this domain of life. The thermoacidophilic crenarchaeon Sulfolobus acidocaldarius harbors a GntR-like regulator belonging to the YtrA subfamily, encoded as the first gene in an operon with a second gene encoding a putative membrane protein. Here, we present a detailed characterization of this regulator, named YtrA Sa , with a focus on regulon determination and mechanistic analysis with regards to DNA binding. Genome-wide chromatin immunoprecipitation and transcriptome experiments, the latter employing a ytrA Sa overexpression strain, demonstrate that the regulator acts as a repressor on a very restricted regulon, consisting of only two targets including the operon encoding its own gene and a distinct genetic locus encoding another putative membrane protein. For both targets, a conserved 14-bp semi-palindromic binding motif was delineated that covers the transcriptional start site and that is surrounded by additional half-site motifs. The crystallographic structure of YtrA Sa was determined, revealing a compact dimeric structure in which the DNA-binding motifs are oriented ideally to enable a specific high-affinity interaction with the core binding motif. This study provides new insights into the functioning of a YtrA-like regulator in the archaeal domain of life.
Organizational Affiliation:
Research Group of Microbiology, Department of Bioengineering Sciences, Vrije Universiteit Brussel, Brussels, Belgium.