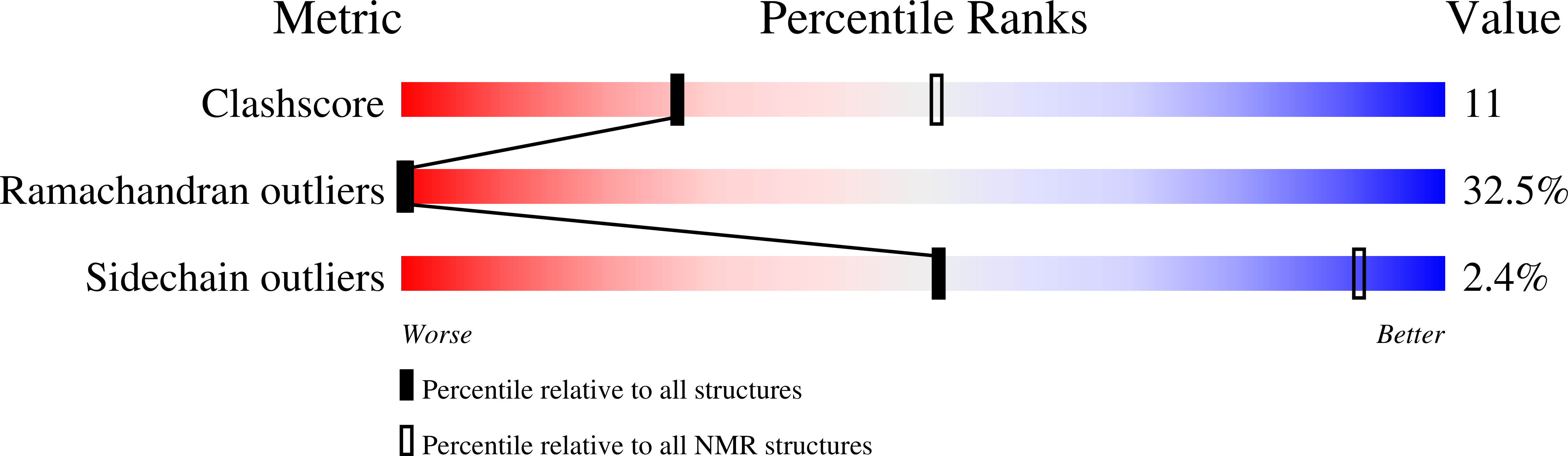A pleurocidin analogue with greater conformational flexibility, enhanced antimicrobial potency and in vivo therapeutic efficacy.
Manzo, G., Hind, C.K., Ferguson, P.M., Amison, R.T., Hodgson-Casson, A.C., Ciazynska, K.A., Weller, B.J., Clarke, M., Lam, C., Man, R.C.H., Shaughnessy, B.G.O., Clifford, M., Bui, T.T., Drake, A.F., Atkinson, R.A., Lam, J.K.W., Pitchford, S.C., Page, C.P., Phoenix, D.A., Lorenz, C.D., Sutton, J.M., Mason, A.J.(2020) Commun Biol 3: 697-697
- PubMed: 33247193
- DOI: https://doi.org/10.1038/s42003-020-01420-3
- Primary Citation of Related Structures:
6RSF, 6RSG - PubMed Abstract:
Antimicrobial peptides (AMPs) are a potential alternative to classical antibiotics that are yet to achieve a therapeutic breakthrough for treatment of systemic infections. The antibacterial potency of pleurocidin, an AMP from Winter Flounder, is linked to its ability to cross bacterial plasma membranes and seek intracellular targets while also causing membrane damage. Here we describe modification strategies that generate pleurocidin analogues with substantially improved, broad spectrum, antibacterial properties, which are effective in murine models of bacterial lung infection. Increasing peptide-lipid intermolecular hydrogen bonding capabilities enhances conformational flexibility, associated with membrane translocation, but also membrane damage and potency, most notably against Gram-positive bacteria. This negates their ability to metabolically adapt to the AMP threat. An analogue comprising D-amino acids was well tolerated at an intravenous dose of 15 mg/kg and similarly effective as vancomycin in reducing EMRSA-15 lung CFU. This highlights the therapeutic potential of systemically delivered, bactericidal AMPs.
Organizational Affiliation:
Institute of Pharmaceutical Science, School of Cancer & Pharmaceutical Science, King's College London, Franklin-Wilkins Building, 150 Stamford Street, London, SE1 9NH, UK.