Structural and Functional Analysis of BBA03, Borrelia burgdorferi Competitive Advantage Promoting Outer Surface Lipoprotein.
Fridmanis, J., Bobrovs, R., Brangulis, K., Tars, K., Jaudzems, K.(2020) Pathogens 9
- PubMed: 33050189
- DOI: https://doi.org/10.3390/pathogens9100826
- Primary Citation of Related Structures:
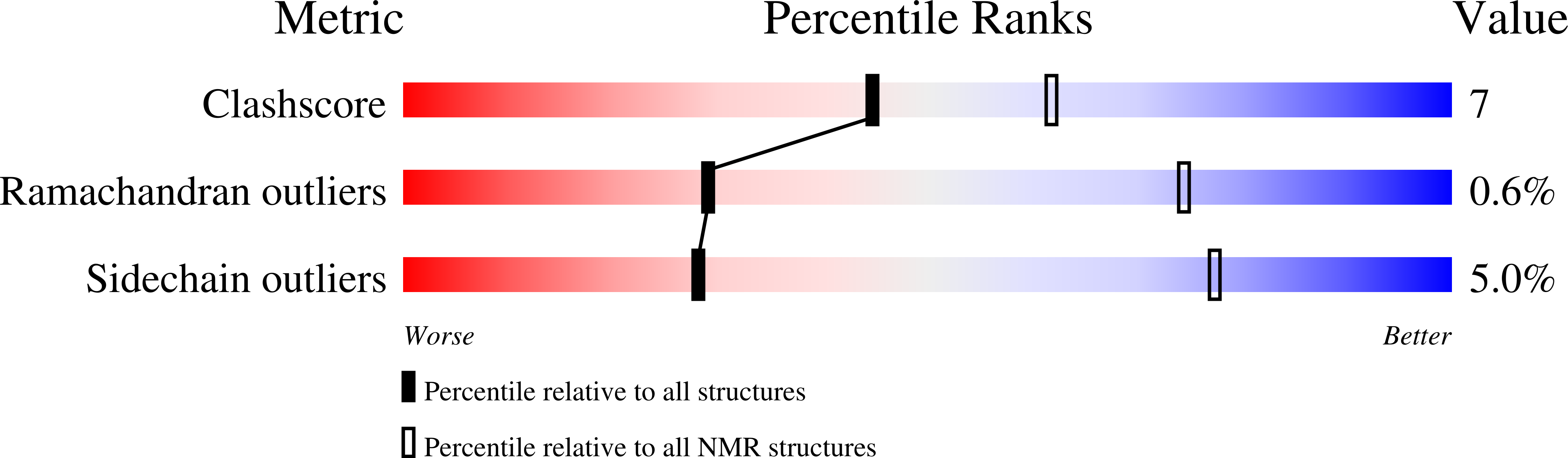
6QS0 - PubMed Abstract:
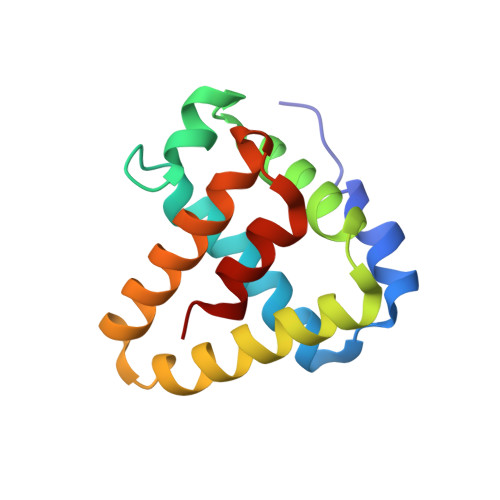
BBA03 is a Borrelia burgdorferi outer surface lipoprotein encoded on one of the most conserved plasmids in Borrelia genome, linear plasmid 54 (lp54). Although many of its genes have been identified as contributing or essential for spirochete fitness in vivo, the majority of the proteins encoded on this plasmid have no known function and lack homologs in other organisms. In this paper, we report the solution NMR structure of the B. burgdorferi outer surface lipoprotein BBA03, which is known to provide a competitive advantage to the bacteria during the transmission from tick vector to mammalian host. BBA03 shows structural homology to other outer surface lipoproteins reflecting their genetic and evolutionary relatedness. Analysis of the structure reveals a pore in BBA03, which could potentially bind lipids.
Organizational Affiliation:
Department of Physical Organic Chemistry, Latvian Institute of Organic Synthesis, Aizkraukles 21, LV-1006 Riga, Latvia.