Expression, purification and crystallization of a novel metagenome-derived salicylaldehyde dehydrogenase from Alpine soil.
Dandare, S.U., Hakansson, M., Svensson, L.A., Timson, D.J., Allen, C.C.R.(2022) Acta Crystallogr F Struct Biol Commun 78: 161-169
- PubMed: 35400668
- DOI: https://doi.org/10.1107/S2053230X22002345
- Primary Citation of Related Structures:
6QHN - PubMed Abstract:
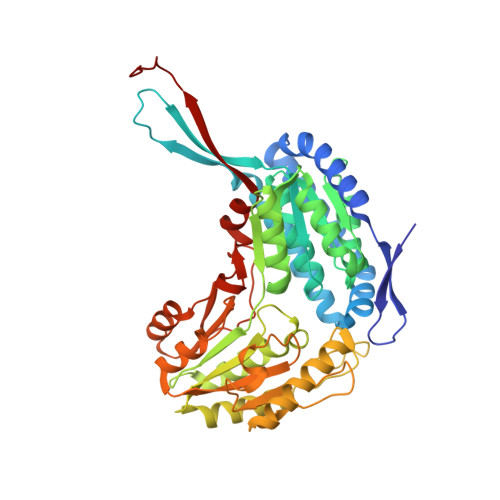
Salicylaldehyde dehydrogenase (SALD) catalyses the last reaction in the upper pathway of naphthalene degradation: the oxidation of salicylaldehyde to salicylate. This enzyme has been isolated and studied from a few organisms that belong to the betaproteobacteria and gammaproteobacteria, predominantly Pseudomonas putida. Furthermore, there is only one crystal structure of this enzyme, which was obtained from P. putida G7. Here, crystallographic studies and analysis of the crystal structure of an Alpine soil metagenome-derived SALD (SALD AP ) from an alphaproteobacterium are presented. The SALD AP gene was discovered using gene-targeted sequence assembly and it was cloned into a pLATE51 vector. The recombinant protein was overexpressed in Escherichia coli BL21 (DE3) cells and the soluble protein was purified to homogeneity. The protein crystallized at 20°C and diffraction data from the crystals were collected at a resolution of 1.9 Å. The crystal belonged to the orthorhombic space group C222 1 , with unit-cell parameters a = 116.8, b = 121.7, c = 318.0 Å. Analysis of the crystal structure revealed its conformation to be similar to the organization of the aldehyde dehydrogenase superfamily with three domains: the catalytic, NAD + -binding and bridging domains. The crystal structure of NahF from P. putida G7 was found to be the best structural homologue of SALD AP , even though the enzymes share only 48% amino-acid identity. Interestingly, a carboxylic acid (protocatechuic acid) was found to be a putative ligand of the enzyme and differential scanning fluorimetry was employed to confirm ligand binding. These findings open up the possibility of studying the mechanism(s) of product inhibition and biocatalysis of carboxylic acids using this enzyme and other related aldehyde dehydrogenases.
Organizational Affiliation:
School of Biological Sciences, Queen's University Belfast, 19 Chlorine Gardens, Belfast BT9 5DL, United Kingdom.