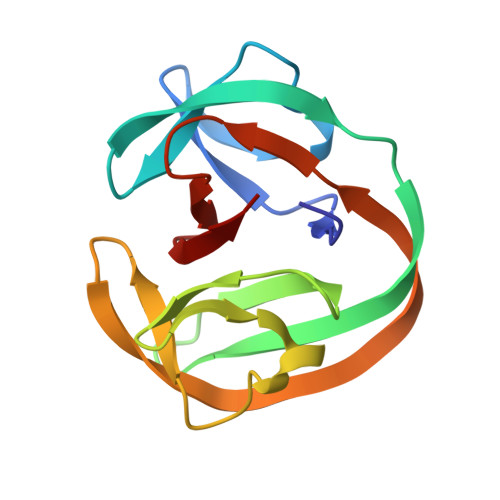
The crystal structure of the naturally split gp41-1 intein guides the engineering of orthogonal split inteins from cis-splicing inteins.
Beyer, H.M., Mikula, K.M., Li, M., Wlodawer, A., Iwai, H.(2020) FEBS J 287: 1886-1898
- PubMed: 31665813
- DOI: https://doi.org/10.1111/febs.15113
- Primary Citation of Related Structures:
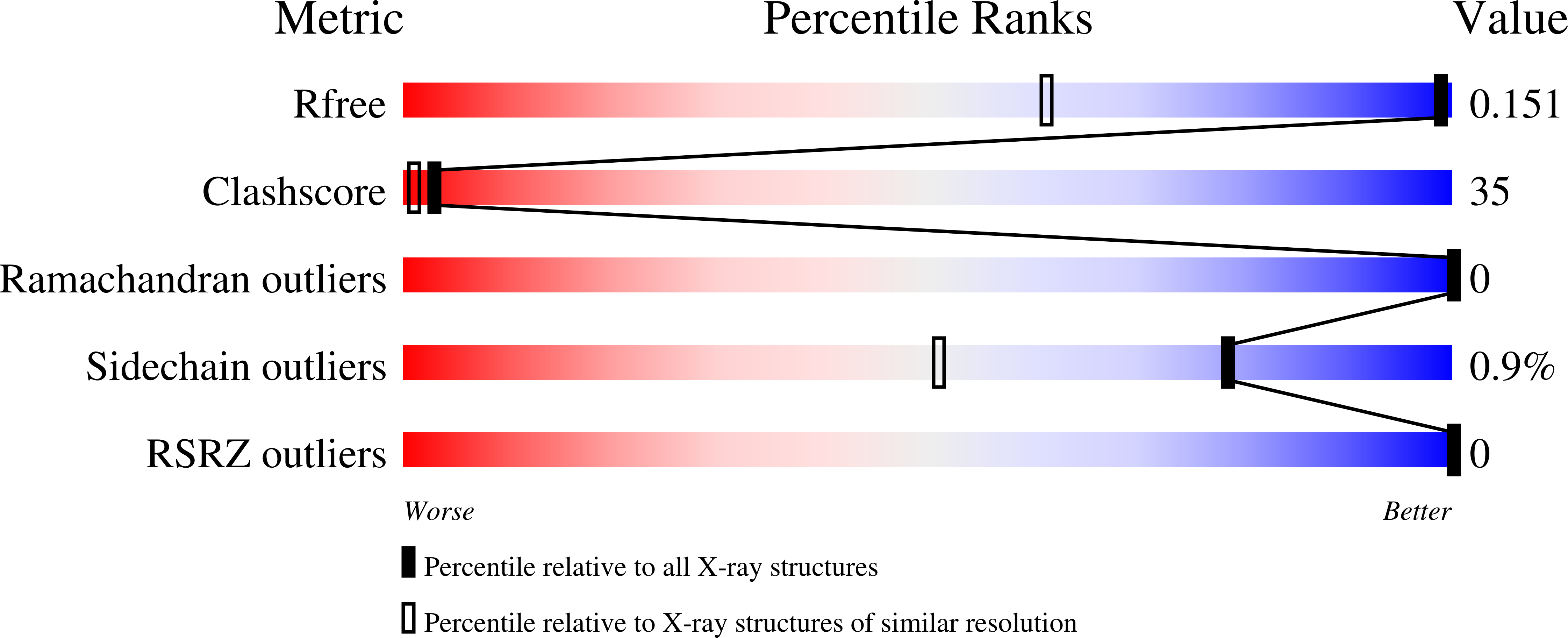
6QAZ - PubMed Abstract:
Protein trans-splicing catalyzed by split inteins has increasingly become useful as a protein engineering tool. We solved the 1.0 Å-resolution crystal structure of a fused variant from the naturally split gp41-1 intein, previously identified from environmental metagenomic sequence data. The structure of the 125-residue gp41-1 intein revealed a compact pseudo-C2-symmetry commonly found in the Hedgehog/Intein superfamily with extensive charge-charge interactions between the split N- and C-terminal intein fragments that are common among naturally occurring split inteins. We successfully created orthogonal split inteins by engineering a similar charge network into the same region of a cis-splicing intein. This strategy could be applicable for creating novel natural-like split inteins from other, more prevalent cis-splicing inteins. DATABASE: Structural data are available in the RCSB Protein Data Bank under the accession number 6QAZ.
Organizational Affiliation:
Research Program in Structural Biology and Biophysics, Helsinki Life Science Institute-Institute of Biotechnology, University of Helsinki, Finland.