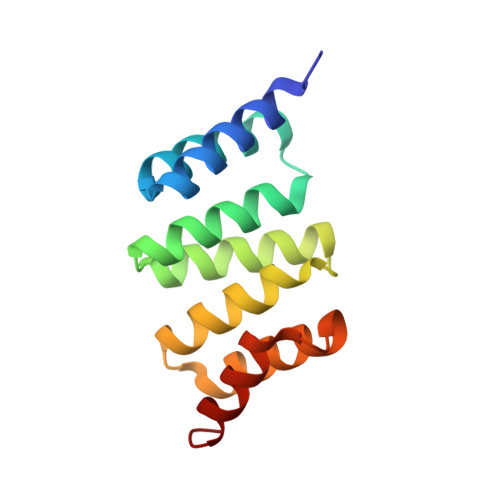
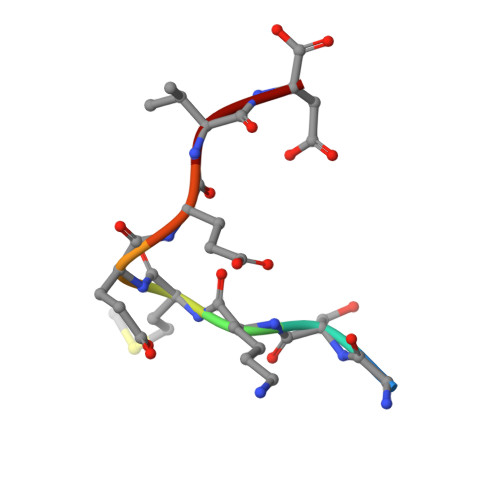
Phosphorylation of the outer membrane mitochondrial protein OM64 influences protein import into mitochondria.
Nickel, C., Horneff, R., Heermann, R., Neumann, B., Jung, K., Soll, J., Schwenkert, S.(2019) Mitochondrion 44: 93-102
- PubMed: 29374544
- DOI: https://doi.org/10.1016/j.mito.2018.01.005
- Primary Citation of Related Structures:
6HPG, 6Q3Q - PubMed Abstract:
Mitochondrial localized proteins are mostly synthesized in the cytosol and translocated across the outer mitochondrial membrane via the translocase of the outer membrane (TOM) complex. Although the channel protein is conserved among eukaryotes, the receptor proteins are more divergent and show features specific to the plant lineage. OM64, which is a paralogue of the chloroplast docking protein Toc64, is unique to plants. However, due to the presence of a cytosolic exposed TPR domain it might functionally replace yeast/mammalian Tom70, which is not found in plant mitochondria, by interacting with the C-terminal (M)EEVD motif of the heat shock proteins Hsp90 and Hsp70. In this study, we show that OM64 is phosphorylated within its TPR domain. Using isothermal titration calorimetry it could be demonstrated that phosphorylation reduces the binding affinity of OM64 to Hsp90. Moreover, in vivo expression of genes encoding different OM64 variants in planta revealed that phosphorylation of OM64 impairs the import efficiency of the mitochondrial preprotein pFAD, a subunits of the mitochondrial ATP synthase. In summary, our data provide significant insight into the fine-tuning mechanisms of mitochondrial protein import mediated by phosphorylation of the cytosolic exposed receptor protein OM64.
Organizational Affiliation:
Munich Center for Integrated Protein Science at the Ludwig-Maximilians-Universität München, Department of Biology I, Botany, Großhaderner Straße 2-4, D-82152 Martinsried, Germany.