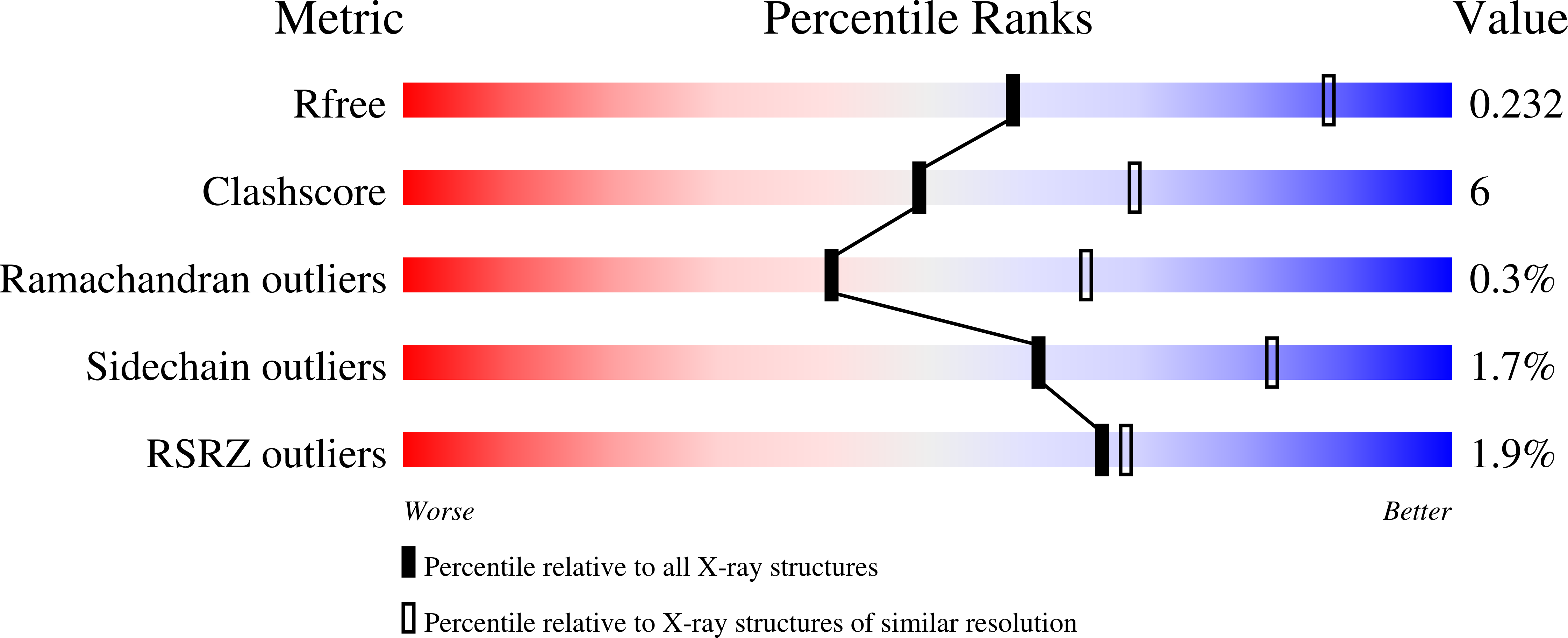
The predominance of nucleotidyl activation in bacterial phosphonate biosynthesis.
Rice, K., Batul, K., Whiteside, J., Kelso, J., Papinski, M., Schmidt, E., Pratasouskaya, A., Wang, D., Sullivan, R., Bartlett, C., Weadge, J.T., Van der Kamp, M.W., Moreno-Hagelsieb, G., Suits, M.D., Horsman, G.P.(2019) Nat Commun 10: 3698-3698
- PubMed: 31420548
- DOI: https://doi.org/10.1038/s41467-019-11627-6
- Primary Citation of Related Structures:
6PD1, 6PD2 - PubMed Abstract:
Phosphonates are rare and unusually bioactive natural products. However, most bacterial phosphonate biosynthetic capacity is dedicated to tailoring cell surfaces with molecules like 2-aminoethylphosphonate (AEP). Although phosphoenolpyruvate mutase (Ppm)-catalyzed installation of C-P bonds is known, subsequent phosphonyl tailoring (Pnt) pathway steps remain enigmatic. Here we identify nucleotidyltransferases in over two-thirds of phosphonate biosynthetic gene clusters, including direct fusions to ~60% of Ppm enzymes. We characterize two putative phosphonyl tailoring cytidylyltransferases (PntCs) that prefer AEP over phosphocholine (P-Cho) - a similar substrate used by the related enzyme LicC, which is a virulence factor in Streptococcus pneumoniae. PntC structural analyses reveal steric discrimination against phosphocholine. These findings highlight nucleotidyl activation as a predominant chemical logic in phosphonate biosynthesis and set the stage for probing diverse phosphonyl tailoring pathways.
Organizational Affiliation:
Department of Chemistry & Biochemistry, Wilfrid Laurier University, Waterloo, ON, N2L 3C5, Canada.