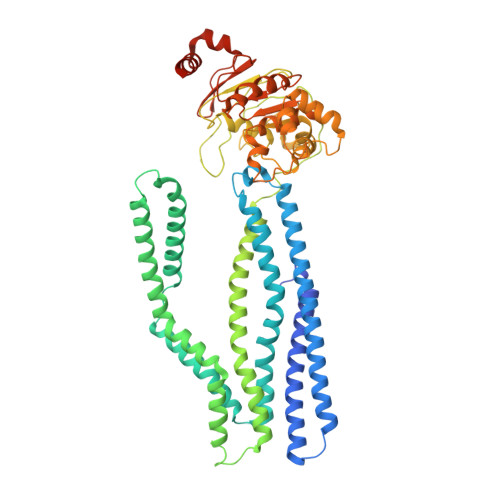
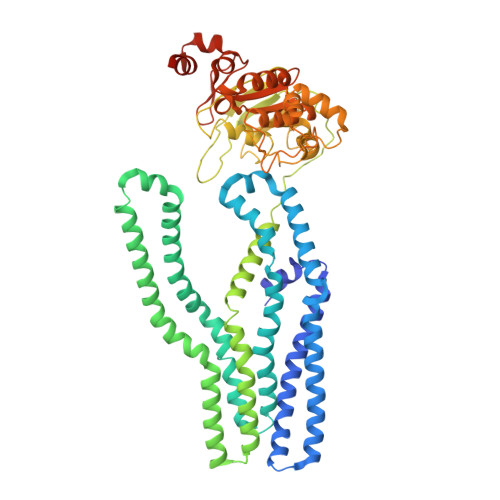
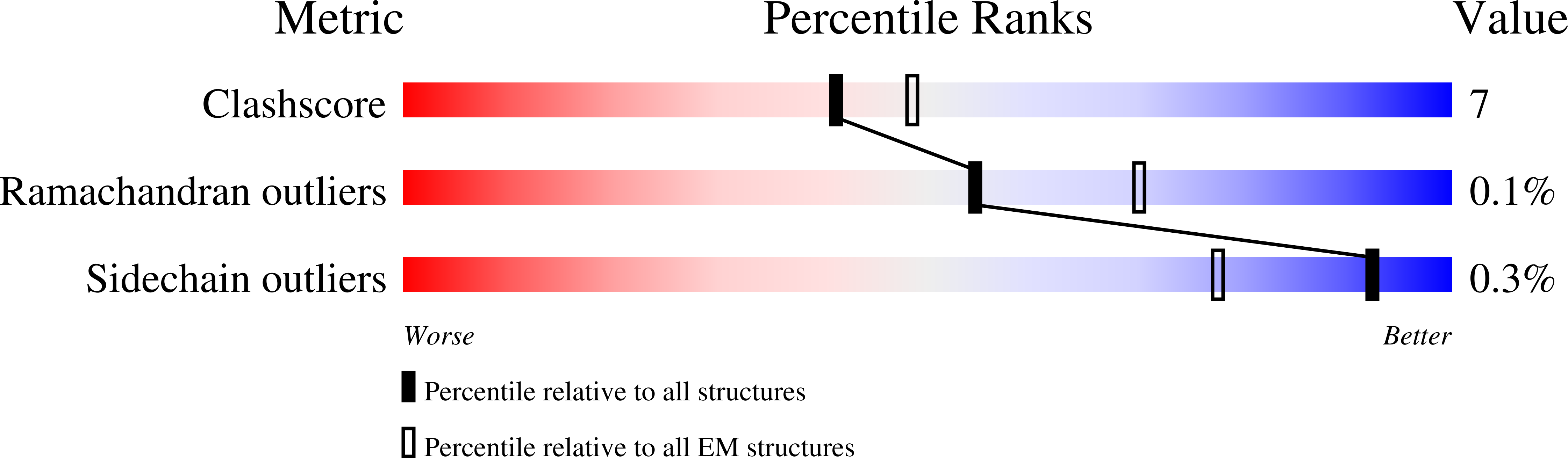Pathogenic siderophore ABC importer YbtPQ adopts a surprising fold of exporter.
Wang, Z., Hu, W., Zheng, H.(2020) Sci Adv 6: eaay7997-eaay7997
- PubMed: 32076651
- DOI: https://doi.org/10.1126/sciadv.aay7997
- Primary Citation of Related Structures:
6P6I, 6P6J - PubMed Abstract:
To fight for essential metal ions, human pathogens secrete virulence-associated siderophores and retake the metal-chelated siderophores through a subfamily of adenosine triphosphate (ATP)-binding cassette (ABC) importer, whose molecular mechanisms are completely unknown. We have determined multiple structures of the yersiniabactin importer YbtPQ from uropathogenic Escherichia coli (UPEC) at inward-open conformation in both apo and substrate-bound states by cryo-electron microscopy. YbtPQ does not adopt any known fold of ABC importers but surprisingly adopts the fold of type IV ABC exporters. To our knowledge, it is the first time an exporter fold of ABC importer has been reported. We have also observed two unique features in YbtPQ: unwinding of a transmembrane helix in YbtP upon substrate release and tightly associated nucleotide-binding domains without bound nucleotides. Together, our study suggests that siderophore ABC importers have a distinct transport mechanism and should be classified as a separate subfamily of ABC importers.
Organizational Affiliation:
Department of Biochemistry and Molecular Genetics, University of Colorado Anschutz Medical Campus, School of Medicine, Aurora, CO, USA.