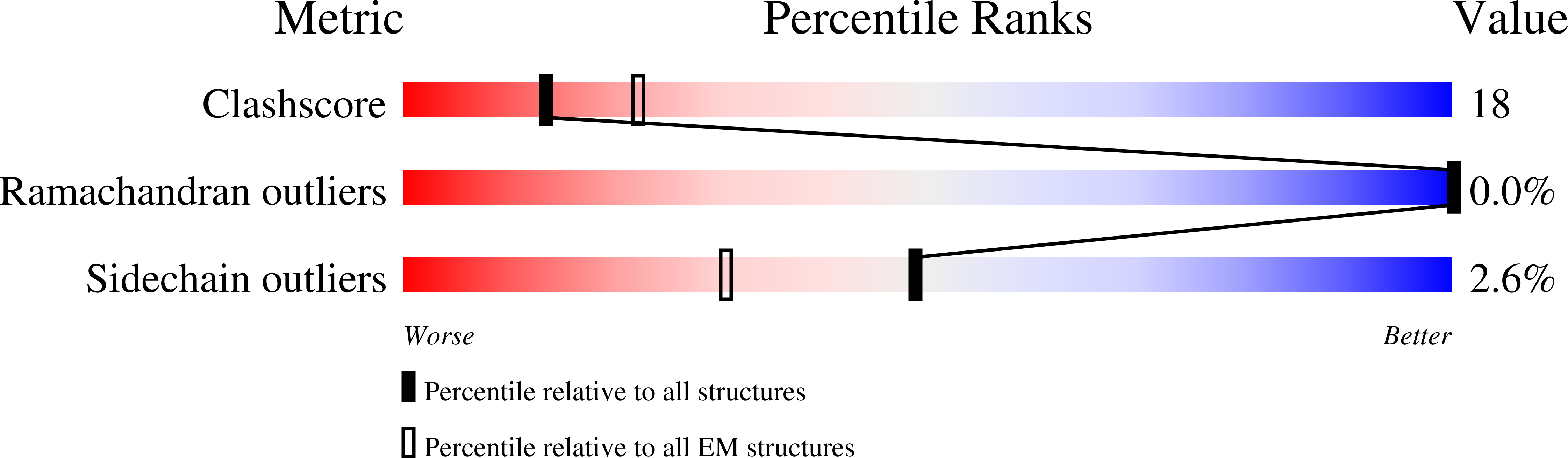Cryo-EM Analysis Reveals Structural Basis of Helicobacter pylori VacA Toxin Oligomerization.
Su, M., Erwin, A.L., Campbell, A.M., Pyburn, T.M., Salay, L.E., Hanks, J.L., Lacy, D.B., Akey, D.L., Cover, T.L., Ohi, M.D.(2019) J Mol Biol 431: 1956-1965
- PubMed: 30954575
- DOI: https://doi.org/10.1016/j.jmb.2019.03.029
- Primary Citation of Related Structures:
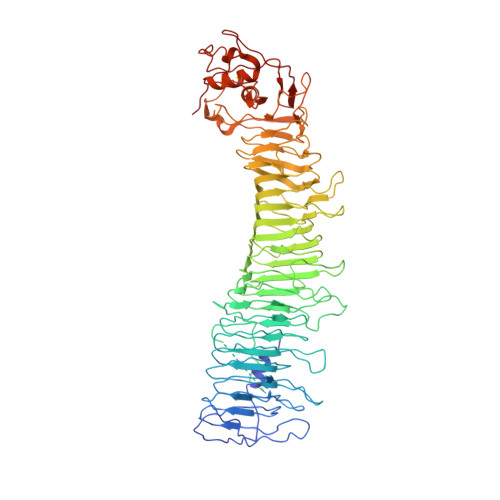
6ODY - PubMed Abstract:
Helicobacter pylori colonizes the human stomach and contributes to the development of gastric cancer and peptic ulcer disease. H. pylori secretes a pore-forming toxin called vacuolating cytotoxin A (VacA), which contains two domains (p33 and p55) and assembles into oligomeric structures. Using single-particle cryo-electron microscopy, we have determined low-resolution structures of a VacA dodecamer and heptamer, as well as a 3.8-Å structure of the VacA hexamer. These analyses show that VacA p88 consists predominantly of a right-handed beta-helix that extends from the p55 domain into the p33 domain. We map the regions of p33 and p55 involved in hexamer assembly, model how interactions between protomers support heptamer formation, and identify surfaces of VacA that likely contact membrane. This work provides structural insights into the process of VacA oligomerization and identifies regions of VacA protomers that are predicted to contact the host cell surface during channel formation.
Organizational Affiliation:
Life Sciences Institute, University of Michigan, Ann Arbor, MI 48109, USA.