Structural and biochemical characterization of the exopolysaccharide deacetylase Agd3 required for Aspergillus fumigatus biofilm formation.
Bamford, N.C., Le Mauff, F., Van Loon, J.C., Ostapska, H., Snarr, B.D., Zhang, Y., Kitova, E.N., Klassen, J.S., Codee, J.D.C., Sheppard, D.C., Howell, P.L.(2020) Nat Commun 11: 2450-2450
- PubMed: 32415073
- DOI: https://doi.org/10.1038/s41467-020-16144-5
- Primary Citation of Related Structures:
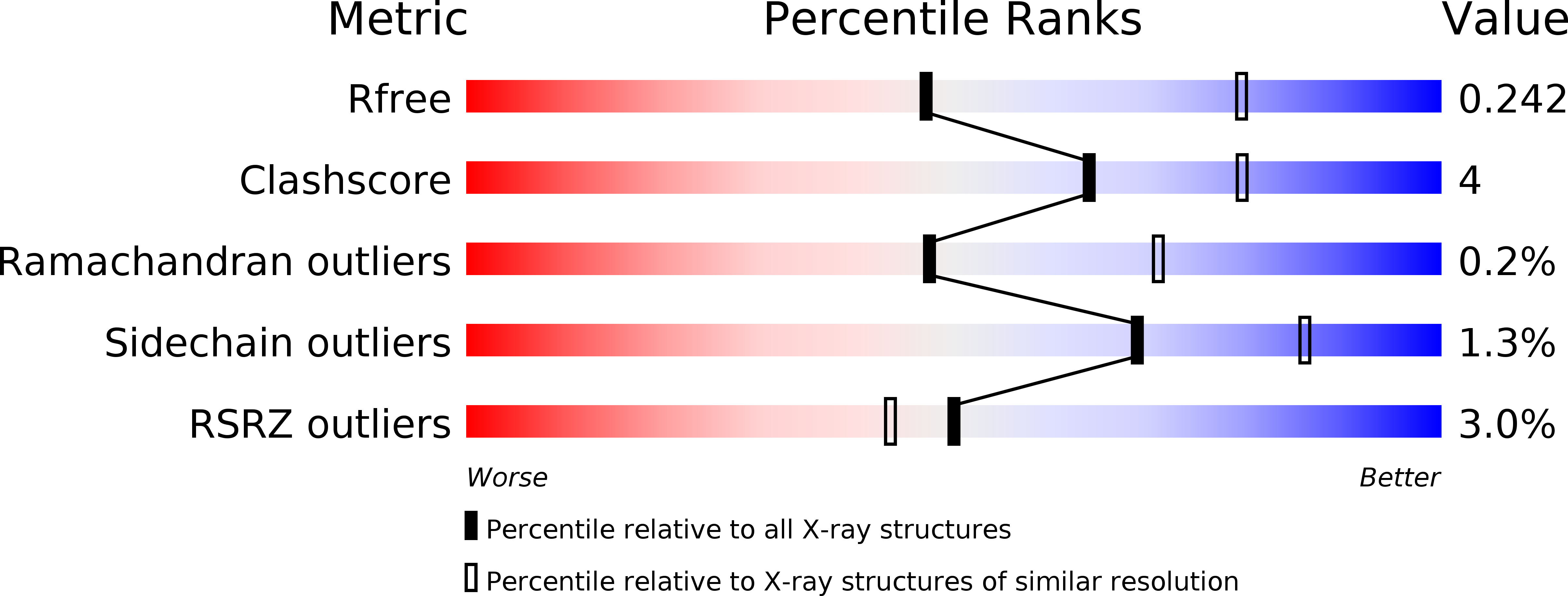
6NWZ - PubMed Abstract:
The exopolysaccharide galactosaminogalactan (GAG) is an important virulence factor of the fungal pathogen Aspergillus fumigatus. Deletion of a gene encoding a putative deacetylase, Agd3, leads to defects in GAG deacetylation, biofilm formation, and virulence. Here, we show that Agd3 deacetylates GAG in a metal-dependent manner, and is the founding member of carbohydrate esterase family CE18. The active site is formed by four catalytic motifs that are essential for activity. The structure of Agd3 includes an elongated substrate-binding cleft formed by a carbohydrate binding module (CBM) that is the founding member of CBM family 87. Agd3 homologues are encoded in previously unidentified putative bacterial exopolysaccharide biosynthetic operons and in other fungal genomes.
Organizational Affiliation:
Program in Molecular Medicine, The Hospital for Sick Children, Toronto, ON, M5G 1X8, Canada.