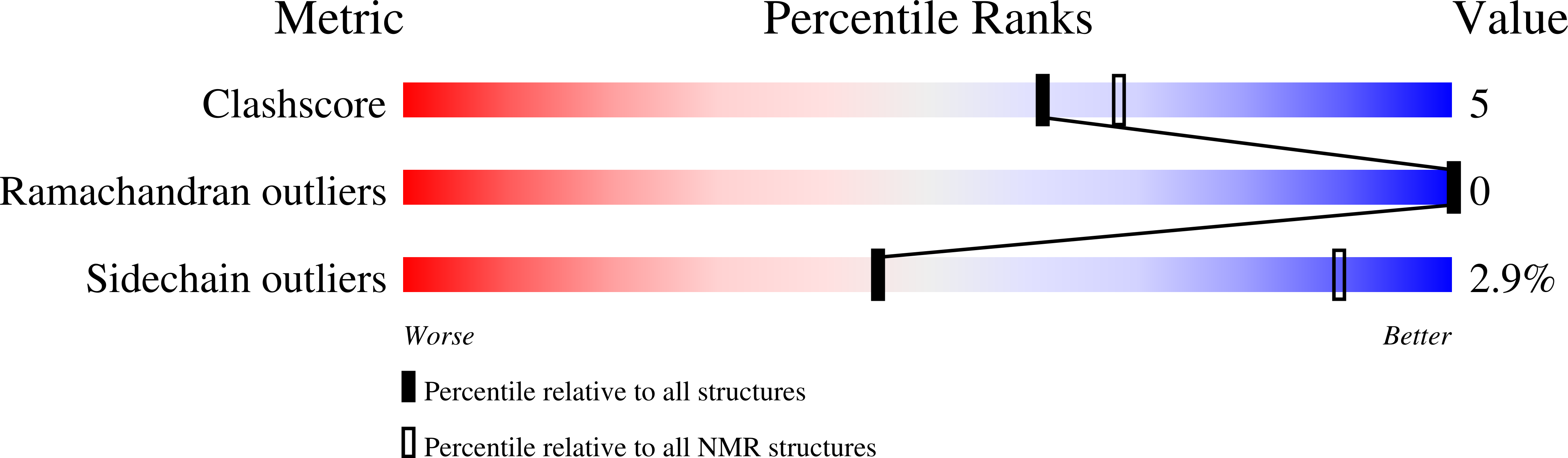Cn29, a novel orphan peptide found in the venom of the scorpion Centruroides noxius: Structure and function.
Gurrola, G.B., Guijarro, J.I., Delepierre, M., Mendoza, R.L.L., Cid-Uribe, J.I., Coronas, F.V., Possani, L.D.(2019) Toxicon 167: 184-191
- PubMed: 31226259
- DOI: https://doi.org/10.1016/j.toxicon.2019.06.013
- Primary Citation of Related Structures:
6NW8 - PubMed Abstract:
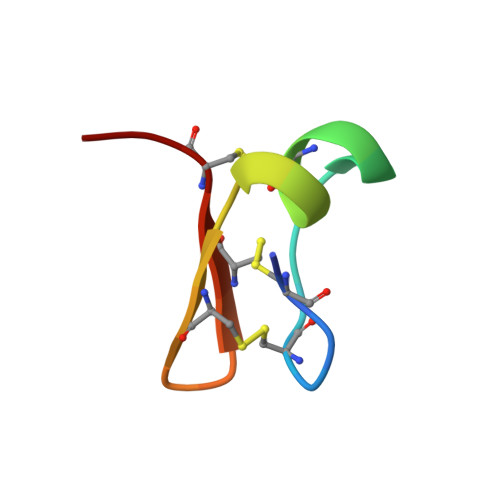
A peptide (Cn29) from the venom of the scorpion Centruroides noxius (about 2% of the soluble venom) was purified and its primary and three-dimensional structures were determined. The peptide contains 27 amino acids with primary sequence: LCLSCRGGDYDCRVKGTCENGKCVCGS. The peptide is tightly packed by three disulfide linkages formed between C2-C23, C5-C18 and C12-C25. Since the native peptide was obtained in limited amounts, the full synthetic peptide was prepared using the standard F-moc-based solid phase synthesis method of Merrifield. The native and synthetic peptides were shown to be identical by sequencing, HPLC separation and mass spectrometry. The solution structure of the peptide solved from NMR data shows that it consists of a well-defined N-terminal region without regular secondary structure extending from Leu 1 to Asp 9, followed by a short helical fragment from Tyr10 to Val14 and two short β strands (Thr17-Glu19 and Lys22-Val24). The primary and tertiary structures of Cn29 are different from all other scorpion peptides described in the literature. Transcriptome analysis of RNA obtained from C. noxius confirmed the expression of a gene coding for Cn29 in its venom gland. Initial experiments were conducted to identify its possible function: lethality tests in mice and insects as well as ion-channel binding using in vitro electrophysiological assays. None of the physiological or biological tests displayed any activity for this peptide, which at present is considered to be another orphan peptide found in scorpion venoms. The peptide is thus the first example of a novel structural component present in scorpion venoms.
Organizational Affiliation:
Department of Molecular Medicine and Bioprocesses, Instituto de Biotecnologia, Universidad Nacional Autonoma de Mexico, Av, Universidad 2001, Col. Chamilpa, Cuernavaca, Morelos, 62210, Mexico.