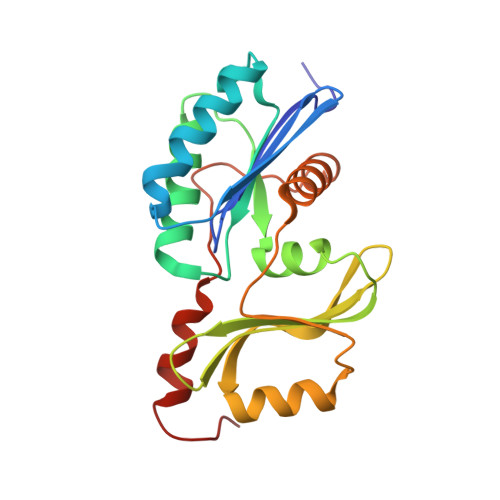
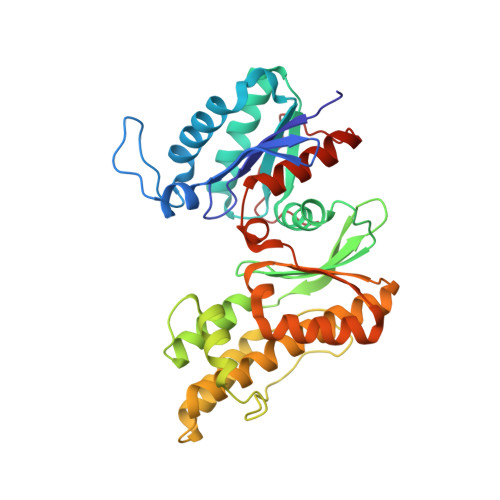
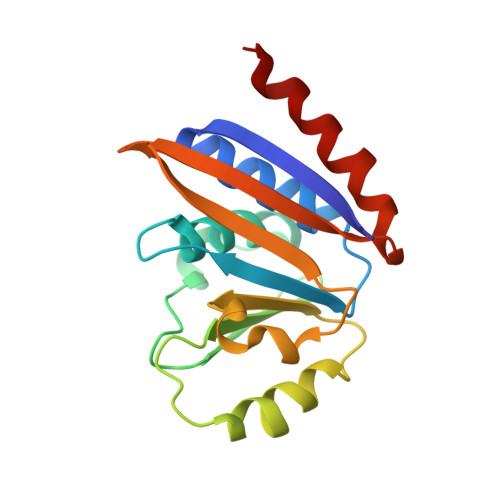
Conformational communication mediates the reset step in t6A biosynthesis.
Luthra, A., Paranagama, N., Swinehart, W., Bayooz, S., Phan, P., Quach, V., Schiffer, J.M., Stec, B., Iwata-Reuyl, D., Swairjo, M.A.(2019) Nucleic Acids Res 47: 6551-6567
- PubMed: 31114923
- DOI: https://doi.org/10.1093/nar/gkz439
- Primary Citation of Related Structures:
6N9A - PubMed Abstract:
The universally conserved N6-threonylcarbamoyladenosine (t6A) modification of tRNA is essential for translational fidelity. In bacteria, t6A biosynthesis starts with the TsaC/TsaC2-catalyzed synthesis of the intermediate threonylcarbamoyl adenylate (TC-AMP), followed by transfer of the threonylcarbamoyl (TC) moiety to adenine-37 of tRNA by the TC-transfer complex comprised of TsaB, TsaD and TsaE subunits and possessing an ATPase activity required for multi-turnover of the t6A cycle. We report a 2.5-Å crystal structure of the T. maritima TC-transfer complex (TmTsaB2D2E2) bound to Mg2+-ATP in the ATPase site, and substrate analog carboxy-AMP in the TC-transfer site. Site directed mutagenesis results show that residues in the conserved Switch I and Switch II motifs of TsaE mediate the ATP hydrolysis-driven reactivation/reset step of the t6A cycle. Further, SAXS analysis of the TmTsaB2D2-tRNA complex in solution reveals bound tRNA lodged in the TsaE binding cavity, confirming our previous biochemical data. Based on the crystal structure and molecular docking of TC-AMP and adenine-37 in the TC-transfer site, we propose a model for the mechanism of TC transfer by this universal biosynthetic system.
Organizational Affiliation:
Department of Chemistry and Biochemistry, San Diego State University, 5500 Campanile Drive, San Diego, CA 92182, USA.