Structural Insights into Bacteriophage GIL01 gp7 Inhibition of Host LexA Repressor.
Caveney, N.A., Pavlin, A., Caballero, G., Bahun, M., Hodnik, V., de Castro, L., Fornelos, N., Butala, M., Strynadka, N.C.J.(2019) Structure 27: 1094
- PubMed: 31056420
- DOI: https://doi.org/10.1016/j.str.2019.03.019
- Primary Citation of Related Structures:
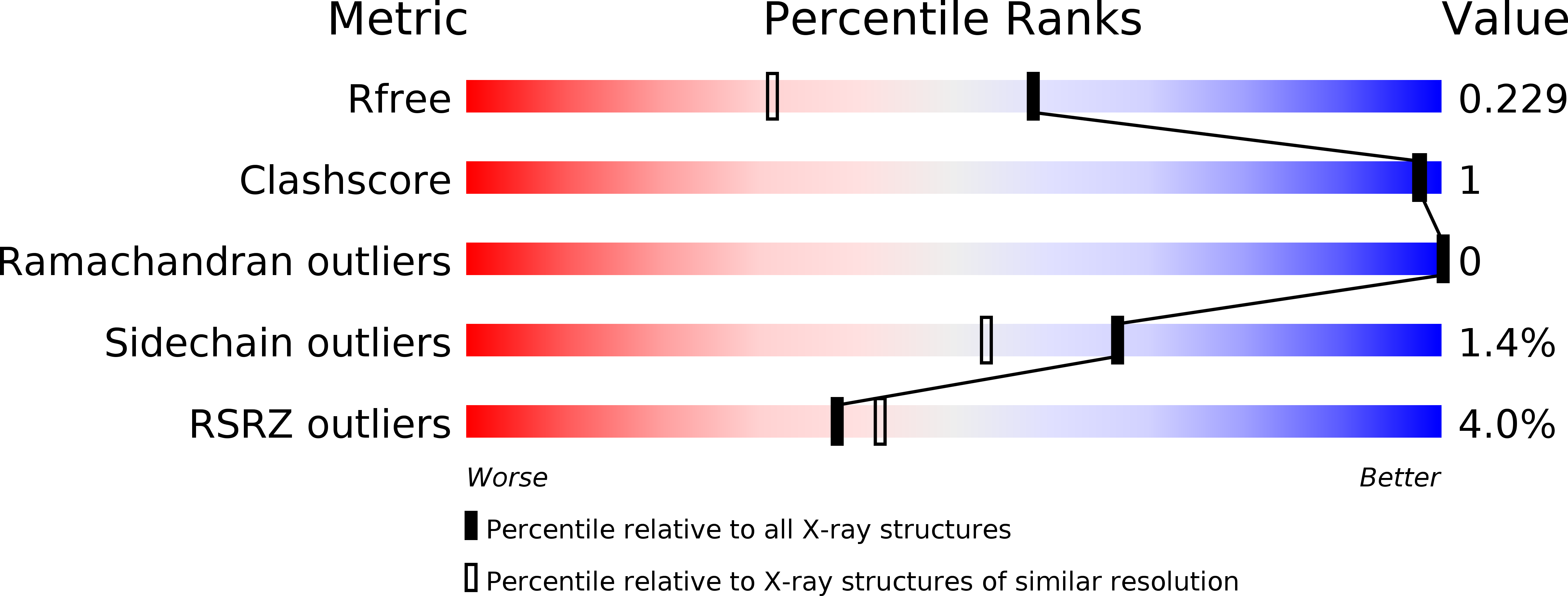
6N7O - PubMed Abstract:
Bacteria identify and respond to DNA damage using the SOS response. LexA, a central repressor in the response, has been implicated in the regulation of lysogeny in various temperate bacteriophages. During infection of Bacillus thuringiensis with GIL01 bacteriophage, LexA represses the SOS response and the phage lytic cycle by binding DNA, an interaction further stabilized upon binding of a viral protein, gp7. Here we report the crystallographic structure of phage-borne gp7 at 1.7-Å resolution, and characterize the 4:2 stoichiometry and potential interaction with LexA using surface plasmon resonance, static light scattering, and small-angle X-ray scattering. These data suggest that gp7 stabilizes LexA binding to operator DNA via coordination of the N- and C-terminal domains of LexA. Furthermore, we have found that gp7 can interact with LexA from Staphylococcus aureus, a significant human pathogen. Our results provide structural evidence as to how phage factors can directly associate with LexA to modulate the SOS response.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology and the Centre for Blood Research, University of British Columbia, Vancouver, BC V6T 1Z3, Canada.