Functional and structural similarities of D7 proteins in the independently-evolved salivary secretions of sand flies and mosquitoes.
Jablonka, W., Kim, I.H., Alvarenga, P.H., Valenzuela, J.G., Ribeiro, J.M.C., Andersen, J.F.(2019) Sci Rep 9: 5340-5340
- PubMed: 30926880
- DOI: https://doi.org/10.1038/s41598-019-41848-0
- Primary Citation of Related Structures:
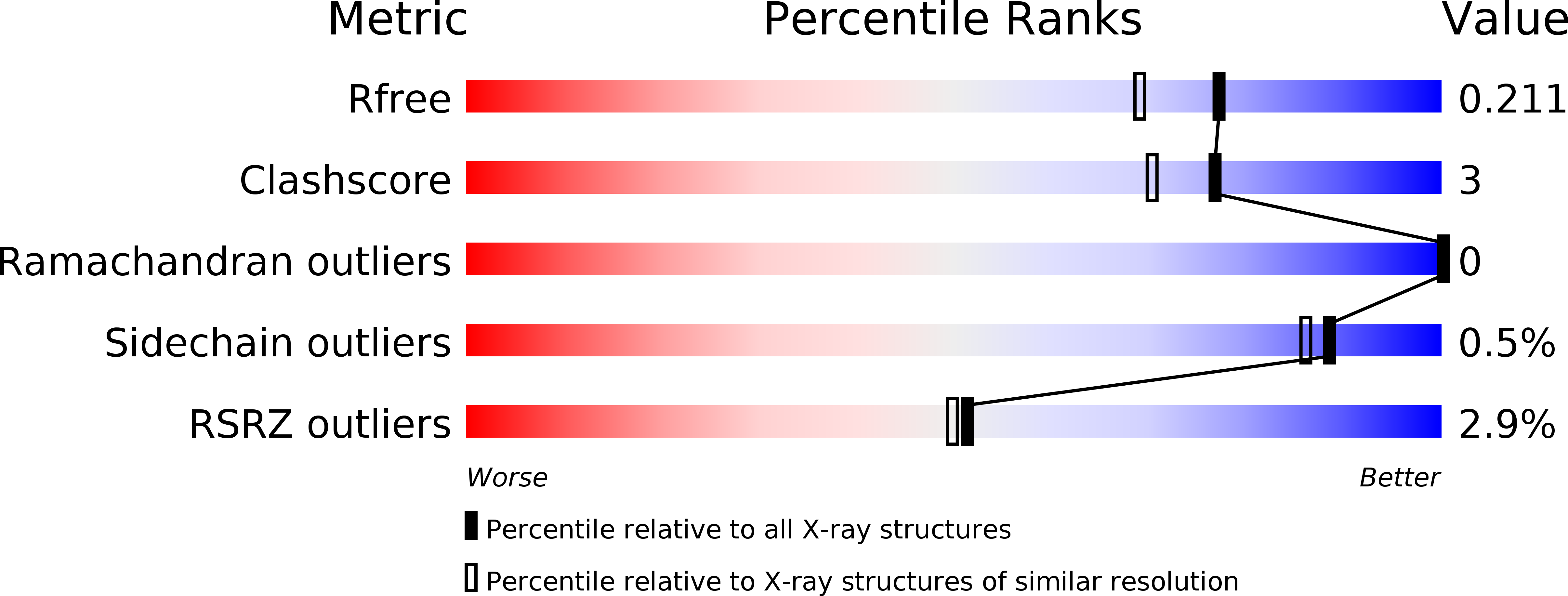
6MT7, 6MTF - PubMed Abstract:
The habit of blood feeding evolved independently in many insect orders of families. Sand flies and mosquitoes belong to separate lineages of blood-feeding Diptera and are thus considered to have evolved the trait independently. Because of this, sand fly salivary proteins differ structurally from those of mosquitoes, and orthologous groups are nearly impossible to define. An exception is the long-form D7-like proteins that show conservation with their mosquito counterparts of numerous residues associated with the N-terminal domain binding pocket. In mosquitoes, this pocket is responsible for the scavenging of proinflammatory cysteinyl leukotrienes and thromboxanes at the feeding site. Here we show that long-form D7 proteins AGE83092 and ABI15936 from the sand fly species, Phlebotomus papatasi and P. duboscqi, respectively, inhibit the activation of platelets by collagen and the thromboxane A 2 analog U46619. Using isothermal titration calorimetry, we also demonstrate direct binding of U46619 and cysteinyl leukotrienes C 4 , D 4 and E 4 to the P. papatasi protein. The crystal structure of P. duboscqi ABI15936 was determined and found to contain two domains oriented similarly to those of the mosquito proteins. The N-terminal domain contains an apparent eicosanoid binding pocket. The C-terminal domain is smaller in overall size than in the mosquito D7s and is missing some helical elements. Consequently, it does not contain an obvious internal binding pocket for small-molecule ligands that bind to many mosquito D7s. Structural similarities indicate that mosquito and sand fly D7 proteins have evolved from similar progenitors, but phylogenetics and differences in intron/exon structure suggest that they may have acquired the ability to bind vertebrate eicosanoids independently, indicating a convergent evolution scenario.
Organizational Affiliation:
The Laboratory of Malaria and Vector Research, NIAID, National Institutes of Health, Rockville, Maryland, 20852, USA.