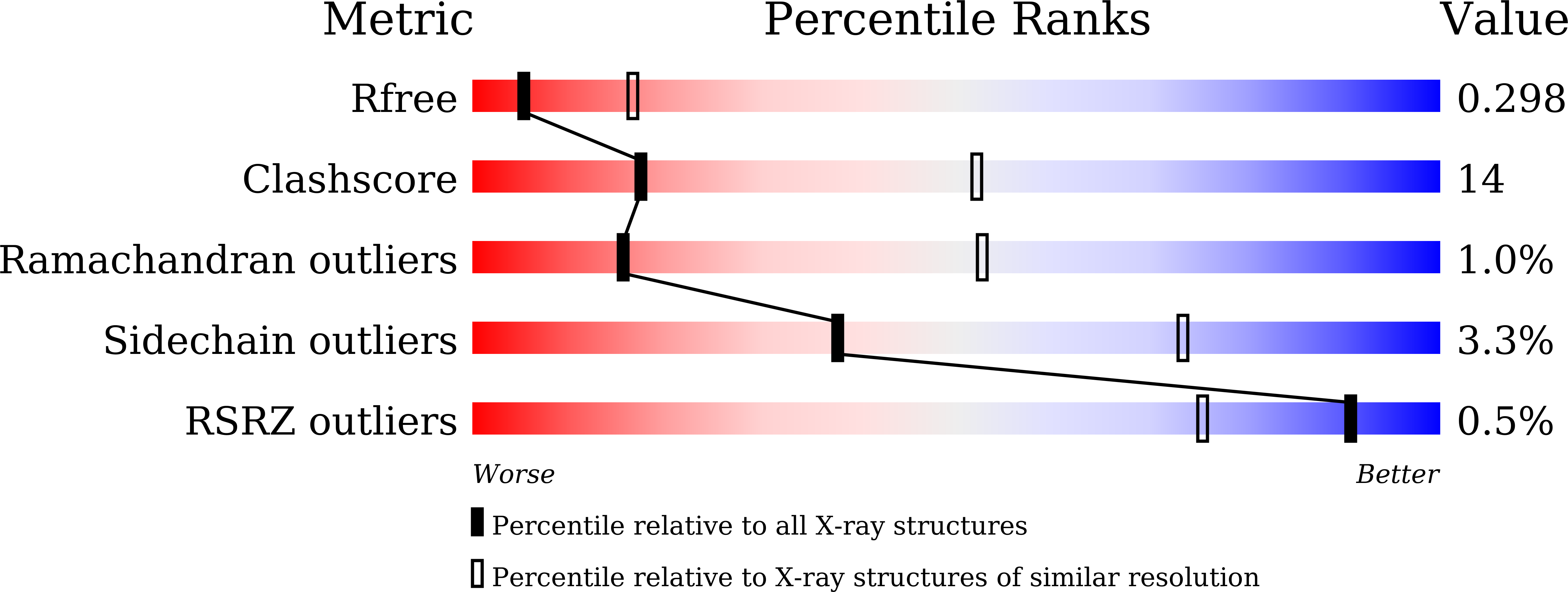
Crystal structure of a GH1 beta-glucosidase from Hamamotoa singularis.
Uehara, R., Iwamoto, R., Aoki, S., Yoshizawa, T., Takano, K., Matsumura, H., Tanaka, S.I.(2020) Protein Sci 29: 2000-2008
- PubMed: 32713015
- DOI: https://doi.org/10.1002/pro.3916
- Primary Citation of Related Structures:
6M4E, 6M4F, 6M55 - PubMed Abstract:
A GH1 β-glucosidase from the fungus Hamamotoa singularis (HsBglA) has high transgalactosylation activity and efficiently converts lactose to galactooligosaccharides. Consequently, HsBglA is among the most widely used enzymes for industrial galactooligosaccharide production. Here, we present the first crystal structures of HsBglA with and without 4'-galactosyllactose, a tri-galactooligosaccharide, at 3.0 and 2.1 Å resolutions, respectively. These structures reveal details of the structural elements that define the catalytic activity and substrate binding of HsBglA, and provide a possible interpretation for its high catalytic potency for transgalactosylation reaction.
Organizational Affiliation:
Department of Biotechnology, College of Life Sciences, Ritsumeikan University, Shiga, Japan.