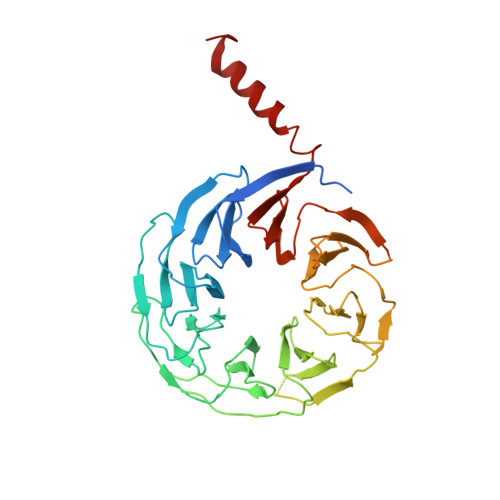
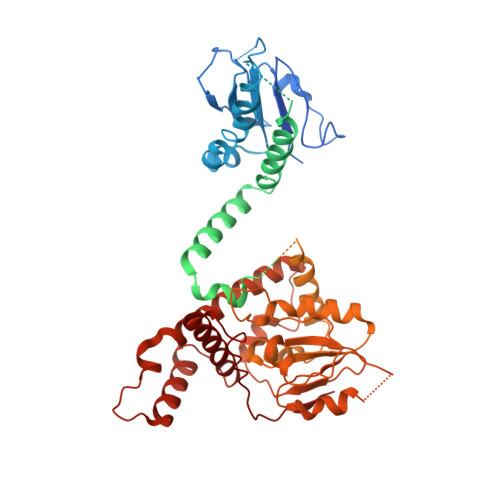
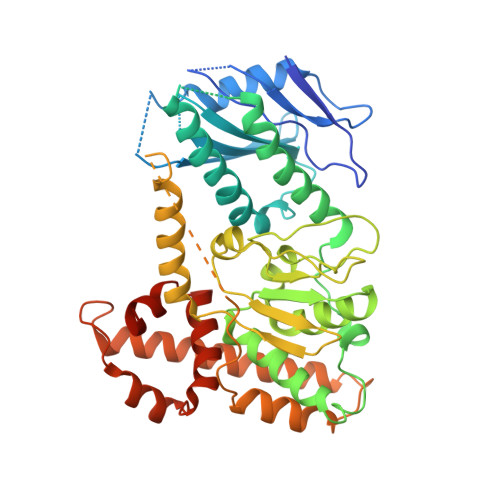
Cryo-EM structure of C9ORF72-SMCR8-WDR41 reveals the role as a GAP for Rab8a and Rab11a.
Tang, D., Sheng, J., Xu, L., Zhan, X., Liu, J., Jiang, H., Shu, X., Liu, X., Zhang, T., Jiang, L., Zhou, C., Li, W., Cheng, W., Li, Z., Wang, K., Lu, K., Yan, C., Qi, S.(2020) Proc Natl Acad Sci U S A 117: 9876-9883
- PubMed: 32303654
- DOI: https://doi.org/10.1073/pnas.2002110117
- Primary Citation of Related Structures:
6LT0 - PubMed Abstract:
A massive intronic hexanucleotide repeat (GGGGCC) expansion in C9ORF72 is a genetic origin of amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD). Recently, C9ORF72, together with SMCR8 and WDR41, has been shown to regulate autophagy and function as Rab GEF. However, the precise function of C9ORF72 remains unclear. Here, we report the cryogenic electron microscopy (cryo-EM) structure of the human C9ORF72-SMCR8-WDR41 complex at a resolution of 3.2 Å. The structure reveals the dimeric assembly of a heterotrimer of C9ORF72-SMCR8-WDR41. Notably, the C-terminal tail of C9ORF72 and the DENN domain of SMCR8 play critical roles in the dimerization of the two protomers of the C9ORF72-SMCR8-WDR41 complex. In the protomer, C9ORF72 and WDR41 are joined by SMCR8 without direct interaction. WDR41 binds to the DENN domain of SMCR8 by the C-terminal helix. Interestingly, the prominent structural feature of C9ORF72-SMCR8 resembles that of the FLNC-FNIP2 complex, the GTPase activating protein (GAP) of RagC/D. Structural comparison and sequence alignment revealed that Arg147 of SMCR8 is conserved and corresponds to the arginine finger of FLCN, and biochemical analysis indicated that the Arg147 of SMCR8 is critical to the stimulatory effect of the C9ORF72-SMCR8 complex on Rab8a and Rab11a. Our study not only illustrates the basis of C9ORF72-SMCR8-WDR41 complex assembly but also reveals the GAP activity of the C9ORF72-SMCR8 complex.
Organizational Affiliation:
Department of Urology, State Key Laboratory of Biotherapy, West China Hospital, College of Life Sciences, Sichuan University, 610041 Chengdu, China.