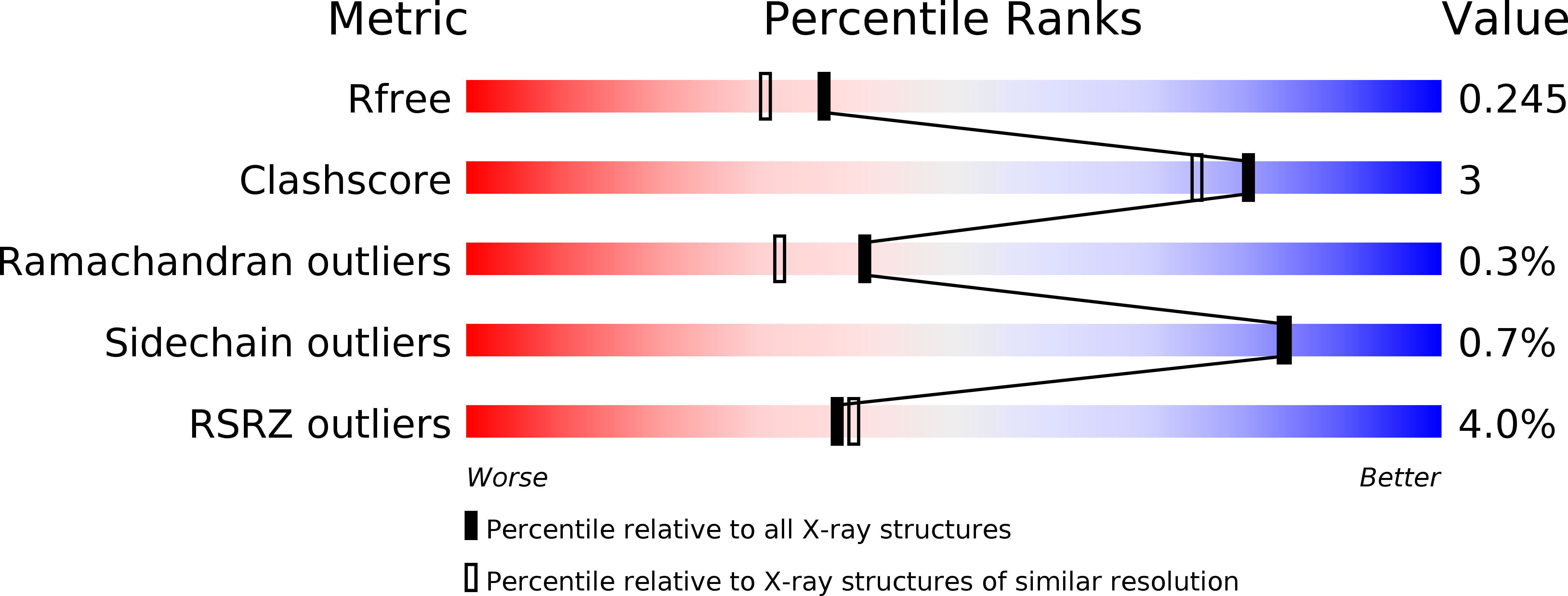
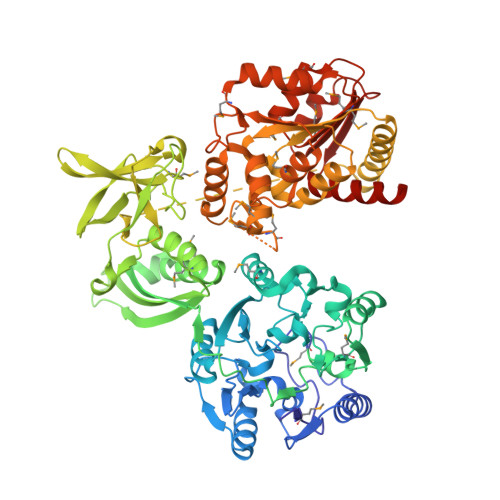
Structural and mutational analyses of the bifunctional arginine dihydrolase and ornithine cyclodeaminase AgrE from the cyanobacteriumAnabaena.
Lee, H., Rhee, S.(2020) J Biol Chem 295: 5751-5760
- PubMed: 32198136
- DOI: https://doi.org/10.1074/jbc.RA120.012768
- Primary Citation of Related Structures:
6LRF, 6LRG, 6LRH - PubMed Abstract:
In cyanobacteria, metabolic pathways that use the nitrogen-rich amino acid arginine play a pivotal role in nitrogen storage and mobilization. The N-terminal domains of two recently identified bacterial enzymes: ArgZ from Synechocystis and AgrE from Anabaena , have been found to contain an arginine dihydrolase. This enzyme provides catabolic activity that converts arginine to ornithine, resulting in concomitant release of CO 2 and ammonia. In Synechocystis , the ArgZ-mediated ornithine-ammonia cycle plays a central role in nitrogen storage and remobilization. The C-terminal domain of AgrE contains an ornithine cyclodeaminase responsible for the formation of proline from ornithine and ammonia production, indicating that AgrE is a bifunctional enzyme catalyzing two sequential reactions in arginine catabolism. Here, the crystal structures of AgrE in three different ligation states revealed that it has a tetrameric conformation, possesses a binding site for the arginine dihydrolase substrate l-arginine and product l-ornithine, and contains a binding site for the coenzyme NAD(H) required for ornithine cyclodeaminase activity. Structure-function analyses indicated that the structure and catalytic mechanism of arginine dihydrolase in AgrE are highly homologous with those of a known bacterial arginine hydrolase. We found that in addition to other active-site residues, Asn-71 is essential for AgrE's dihydrolase activity. Further analysis suggested the presence of a passage for substrate channeling between the two distinct AgrE active sites, which are situated ∼45 Å apart. These results provide structural and functional insights into the bifunctional arginine dihydrolase-ornithine cyclodeaminase enzyme AgrE required for arginine catabolism in Anabaena .
Organizational Affiliation:
Department of Agricultural Biotechnology, Seoul National University, Seoul 151-921, Korea.