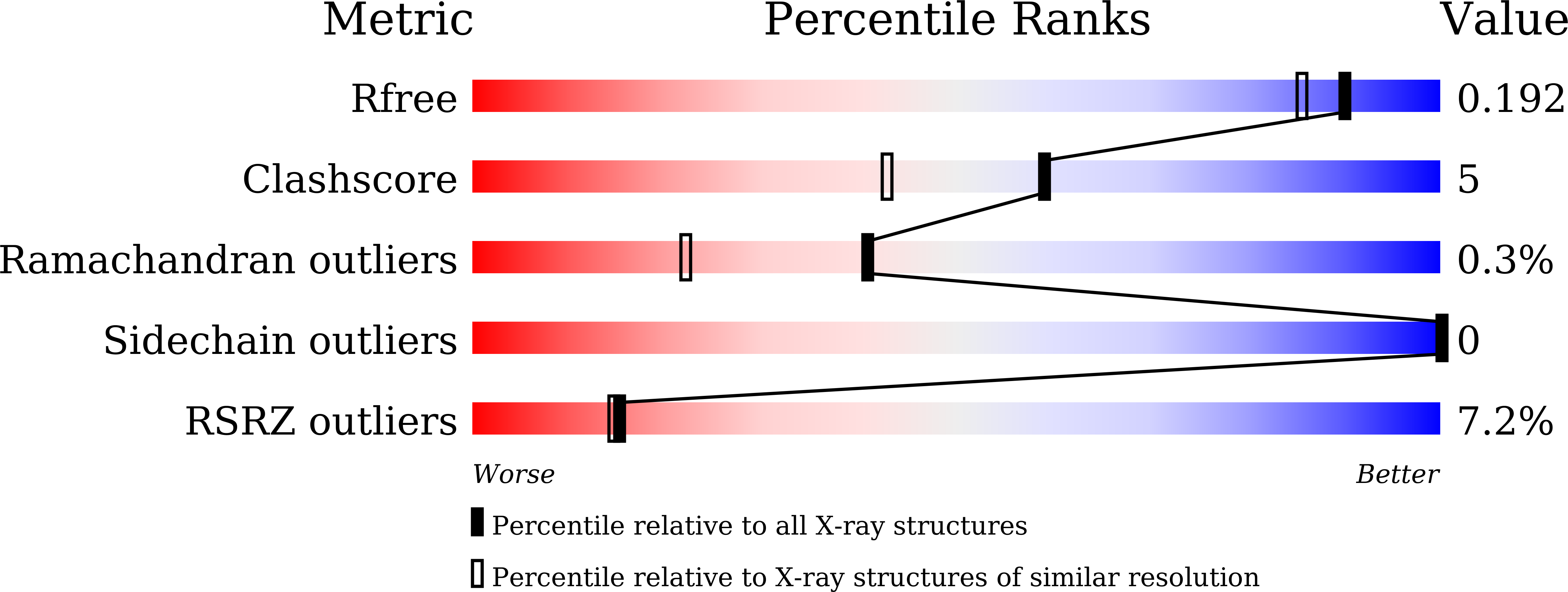
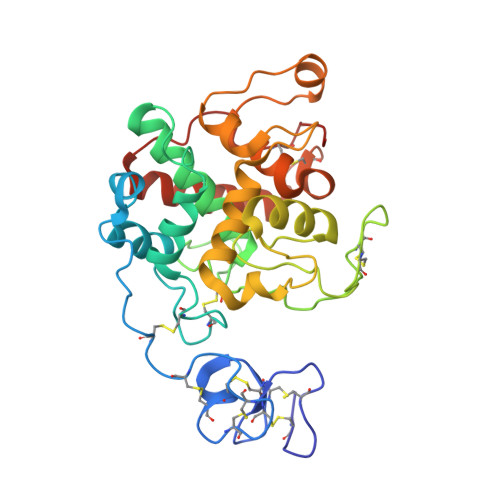
Structure of intact chitinase with hevein domain from the plant Simarouba glauca, known for its traditional anti-inflammatory efficacy.
Balu, K.E., Ramya, K.S., Radha, A., Krishnasamy, G.(2020) Int J Biol Macromol 161: 1381-1392
- PubMed: 32750481
- DOI: https://doi.org/10.1016/j.ijbiomac.2020.07.284
- Primary Citation of Related Structures:
6LNR - PubMed Abstract:
Chitinase from the leaves of Simarouba glauca, a plant used in traditional anti-inflammatory therapy is purified and characterized. Peptide mass finger print analysis revealed the protein as an endo-chitinase which was further confirmed using chitin-agar assay. The enzyme exhibited significant anti-fungal efficacy against phyto-pathogens such as Macrophomina phaseolina, Fusarium oxysporum and Sclerotium rolfsii. Chitinolysis was also examined against insoluble chitin using SEM. Using X-ray diffraction data up to 1.66 Å, the structure was determined by Molecular Replacement using crystal structure of GH19 Chitinase-like protein from Hevea brasiliensis. During structure refinement, an extra domain could be traced and identified as hevein domain. To our knowledge, this is the first report of any chitinase with intact hevein domain. The GH19 chitinase and hevein domains though connected by a lengthy loop, are restricted to be close by disulfide bridges. These bridges connecting each domain with the loop may be important for proper chitin feeding into the active site. By considering reports on hevein and chitinase domains as well as the traditional use of the plant, this report of an intact hevein-chitinase protein and their relative orientation may add further insights for the usefulness of this protein.
Organizational Affiliation:
CAS in Crystallography and Biophysics, University of Madras, Guindy Campus, Chennai 600025, India.