Structural and Biophysical Insights into the TCR alpha beta Complex in Chickens.
Zhang, L., Liu, Y., Meng, G., Liang, R., Zhang, B., Xia, C.(2020) iScience 23: 101828-101828
- PubMed: 33305184
- DOI: https://doi.org/10.1016/j.isci.2020.101828
- Primary Citation of Related Structures:
6LIR - PubMed Abstract:
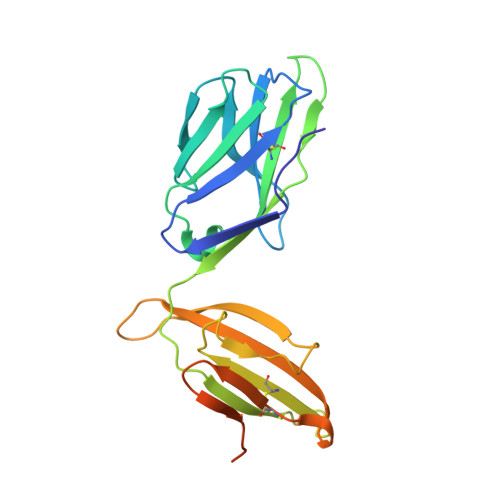
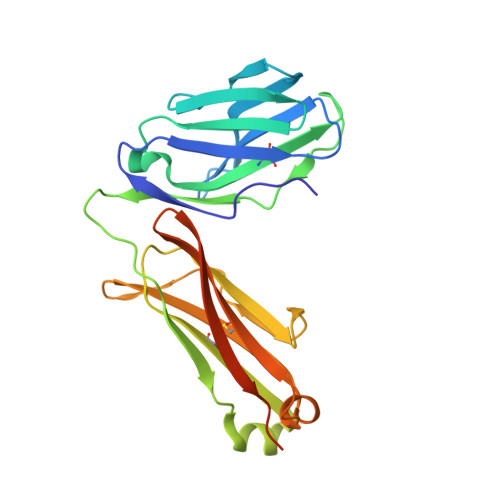
In this work, chicken HPAIV H5N1 epitope-specific TCRαβ (ch-TCRαβ) was isolated and its structure was determined. The Cα domain of ch-TCRαβ does not exhibit the typical structure of human TCRαβ, and the DE loop extends outward, resulting in close proximity between the Cα domain of ch-TCRαβ and CD3εδ/γ. The FG loop of the Cβ domain of ch-TCRαβ is shorter. The changes in the C domains of ch-TCRαβ and the difference in chicken CD3εδ/γ confirm that the complexes formed by TCRαβ and CD3εδ/γ differ from those in humans. In the chicken complex, a positively charged cleft is formed between the two CDR3 loops that might accommodate the acidic side chains of the chicken pMHC-I-bound HPAIV epitope intermediate portion oriented toward ch-TCRαβ. This is the first reported structure of chicken TCRαβ, and it provides a structural model of the ancestral TCR system in the immune synapses between T cells and antigen-presenting cells in lower vertebrates.
Organizational Affiliation:
Department of Microbiology and Immunology, College of Veterinary Medicine, China Agricultural University, Haidian District, Beijing 100193, China.