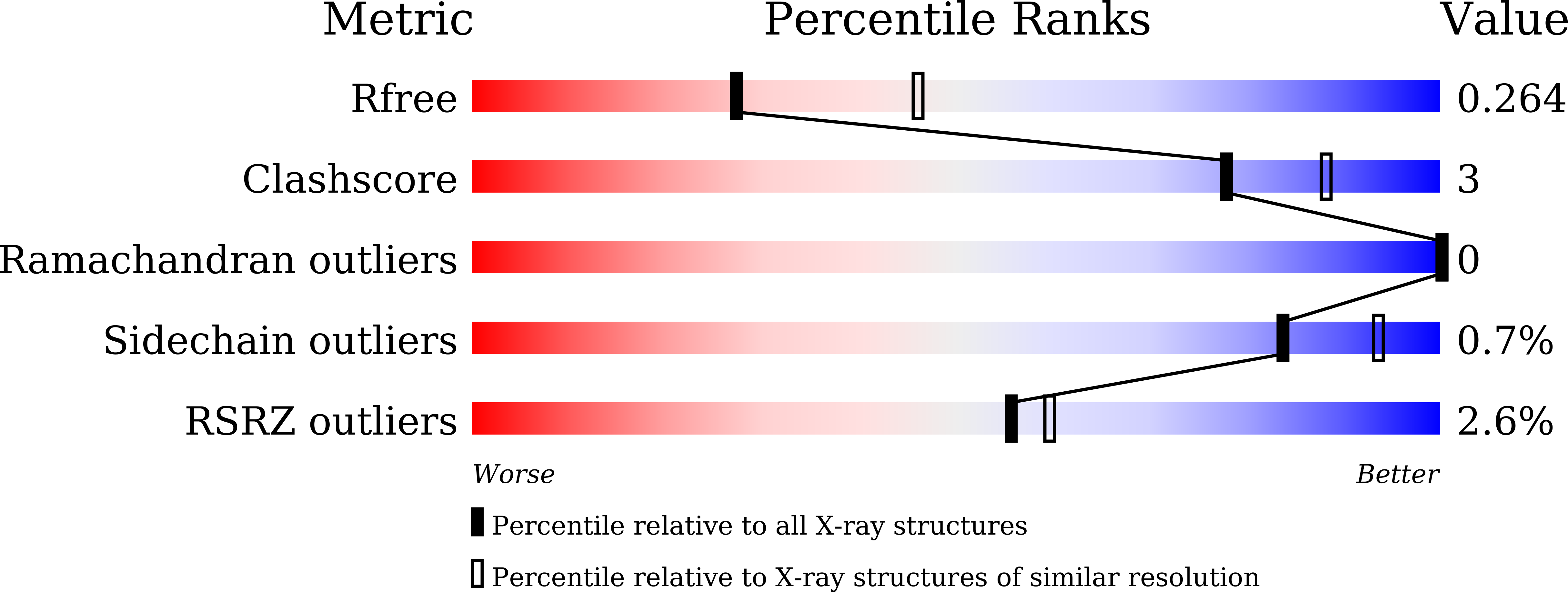
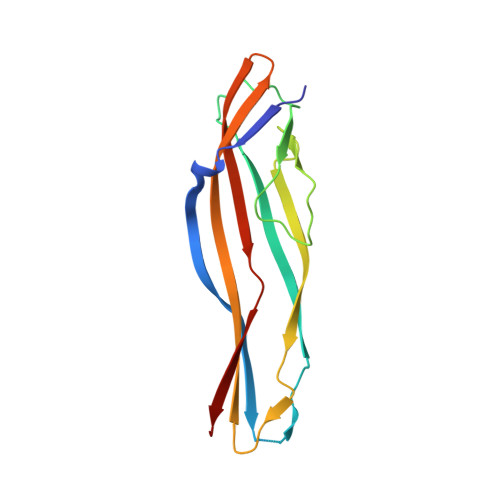
A cellular endolysosome-modulating pore-forming protein from a toad is negatively regulated by its paralog under oxidizing conditions.
Wang, Q., Bian, X., Zeng, L., Pan, F., Liu, L., Liang, J., Wang, L., Zhou, K., Lee, W., Xiang, Y., Li, S., Teng, M., Li, X., Guo, X., Zhang, Y.(2020) J Biol Chem 295: 10293-10306
- PubMed: 32499370
- DOI: https://doi.org/10.1074/jbc.RA120.013556
- Primary Citation of Related Structures:
6LH8, 6LHZ - PubMed Abstract:
Endolysosomes are key players in cell physiology, including molecular exchange, immunity, and environmental adaptation. They are the molecular targets of some pore-forming aerolysin-like proteins (ALPs) that are widely distributed in animals and plants and are functionally related to bacterial toxin aerolysins. βγ-CAT is a complex of an ALP (BmALP1) and a trefoil factor (BmTFF3) in the firebelly toad ( Bombina maxima ). It is the first example of a secreted endogenous pore-forming protein that modulates the biochemical properties of endolysosomes by inducing pore formation in these intracellular vesicles. Here, using a large array of biochemical and cell biology methods, we report the identification of BmALP3, a paralog of BmALP1 that lacks membrane pore-forming capacity. We noted that both BmALP3 and BmALP1 contain a conserved cysteine in their C-terminal regions. BmALP3 was readily oxidized to a disulfide bond-linked homodimer, and this homodimer then oxidized BmALP1 via disulfide bond exchange, resulting in the dissociation of βγ-CAT subunits and the elimination of biological activity. Consistent with its behavior in vitro , BmALP3 sensed environmental oxygen tension in vivo , leading to modulation of βγ-CAT activity. Interestingly, we found that this C-terminal cysteine site is well conserved in numerous vertebrate ALPs. These findings uncover the existence of a regulatory ALP (BmALP3) that modulates the activity of an active ALP (BmALP1) in a redox-dependent manner, a property that differs from those of bacterial toxin aerolysins.
Organizational Affiliation:
Key Laboratory of Animal Models and Human Disease Mechanisms of the Chinese Academy of Sciences/Key Laboratory of Bioactive Peptides of Yunnan Province, Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming, Yunnan, China.