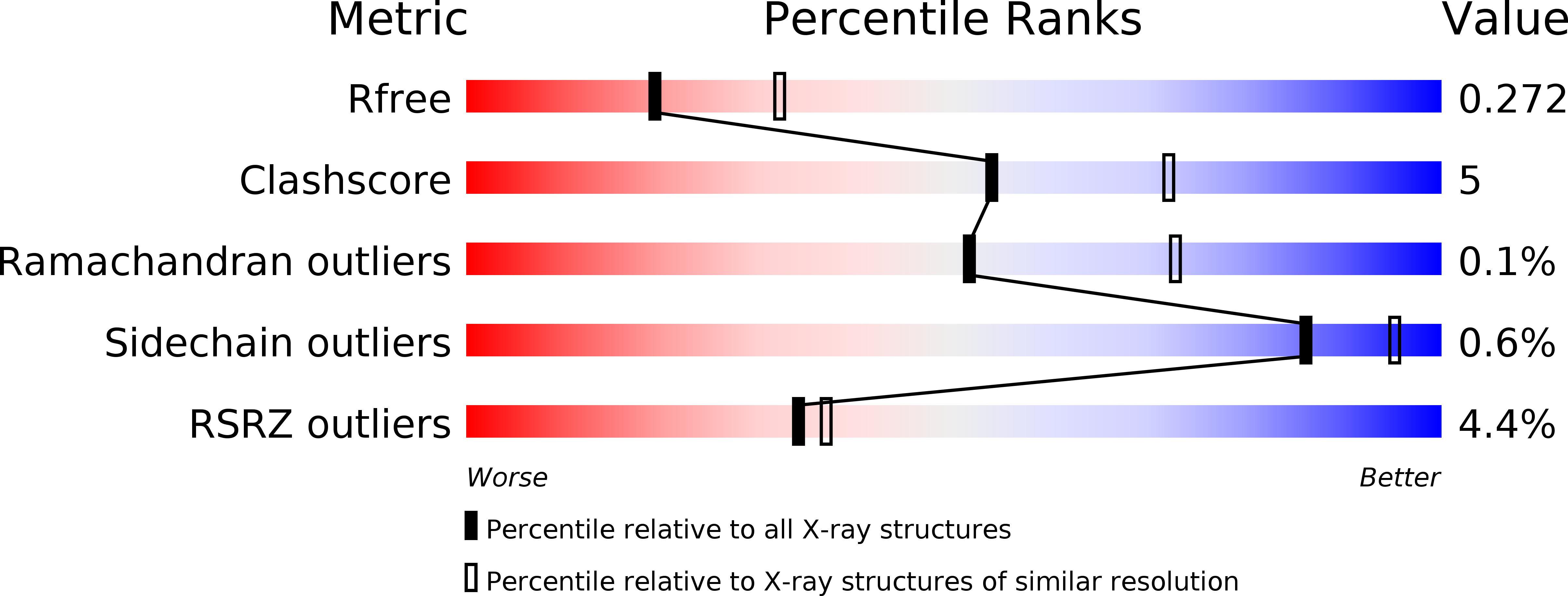
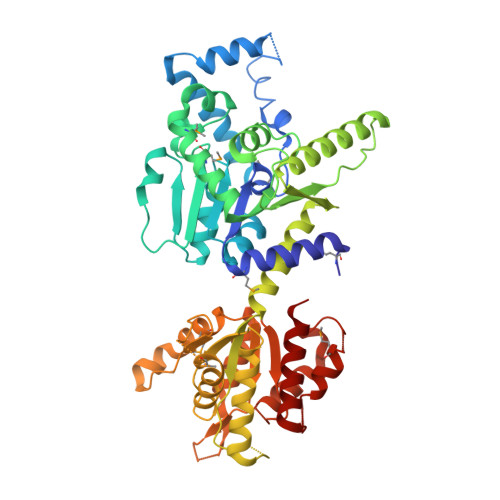
Structural and functional evidence of bacterial antiphage protection by Thoeris defense system via NAD+degradation.
Ka, D., Oh, H., Park, E., Kim, J.H., Bae, E.(2020) Nat Commun 11: 2816-2816
- PubMed: 32499527
- DOI: https://doi.org/10.1038/s41467-020-16703-w
- Primary Citation of Related Structures:
6LHX, 6LHY - PubMed Abstract:
The intense arms race between bacteria and phages has led to the development of diverse antiphage defense systems in bacteria. Unlike well-known restriction-modification and CRISPR-Cas systems, recently discovered systems are poorly characterized. One such system is the Thoeris defense system, which consists of two genes, thsA and thsB. Here, we report structural and functional analyses of ThsA and ThsB. ThsA exhibits robust NAD + cleavage activity and a two-domain architecture containing sirtuin-like and SLOG-like domains. Mutation analysis suggests that NAD + cleavage is linked to the antiphage function of Thoeris. ThsB exhibits a structural resemblance to TIR domain proteins such as nucleotide hydrolases and Toll-like receptors, but no enzymatic activity is detected in our in vitro assays. These results further our understanding of the molecular mechanism underlying the Thoeris defense system, highlighting a unique strategy for bacterial antiphage resistance via NAD + degradation.
Organizational Affiliation:
Department of Agricultural Biotechnology, Seoul National University, Seoul, 08826, Korea.