Understanding Mechanics of competitive-allostery Using Engineered Cysteine Synthase Assembly
Kumar, N., Singh, R.P., Kumaran, S.To be published.
Experimental Data Snapshot
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Serine O-acetyltransferase | 331 | Planctopirus limnophila DSM 3776 | Mutation(s): 0 Gene Names: Plim_1307 EC: 2.3.1.30 | 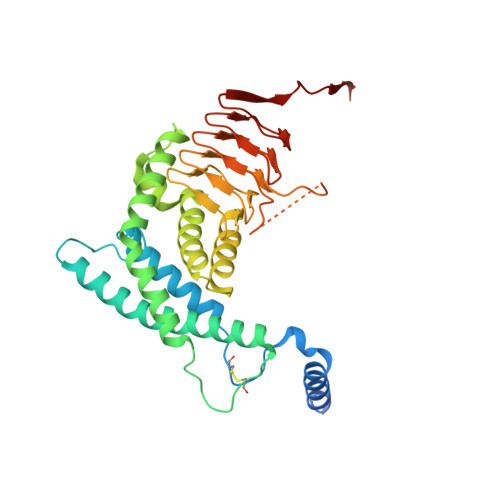 | |
UniProt | |||||
Find proteins for D5SUT9 (Planctopirus limnophila (strain ATCC 43296 / DSM 3776 / IFAM 1008 / Mu 290)) Explore D5SUT9 Go to UniProtKB: D5SUT9 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | D5SUT9 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 3 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| PEG (Subject of Investigation/LOI) Query on PEG | J [auth B], K [auth C], O [auth D], S [auth F] | DI(HYDROXYETHYL)ETHER C4 H10 O3 MTHSVFCYNBDYFN-UHFFFAOYSA-N |  | ||
| GOL (Subject of Investigation/LOI) Query on GOL | G [auth A] H [auth A] L [auth C] N [auth D] Q [auth E] | GLYCEROL C3 H8 O3 PEDCQBHIVMGVHV-UHFFFAOYSA-N |  | ||
| CL (Subject of Investigation/LOI) Query on CL | I [auth A], M [auth C], P [auth D] | CHLORIDE ION Cl VEXZGXHMUGYJMC-UHFFFAOYSA-M |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 84.719 | α = 90 |
| b = 113.165 | β = 90 |
| c = 230.133 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| PHENIX | refinement |
| XDS | data reduction |
| XDS | data scaling |
| PHENIX | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Department of Health & Human Services (HHS) | India | No.3/1/3/JRF-2015/HRD-LS/48/30922/131 |
| Council of Scientific & Industrial Research | India | -- |