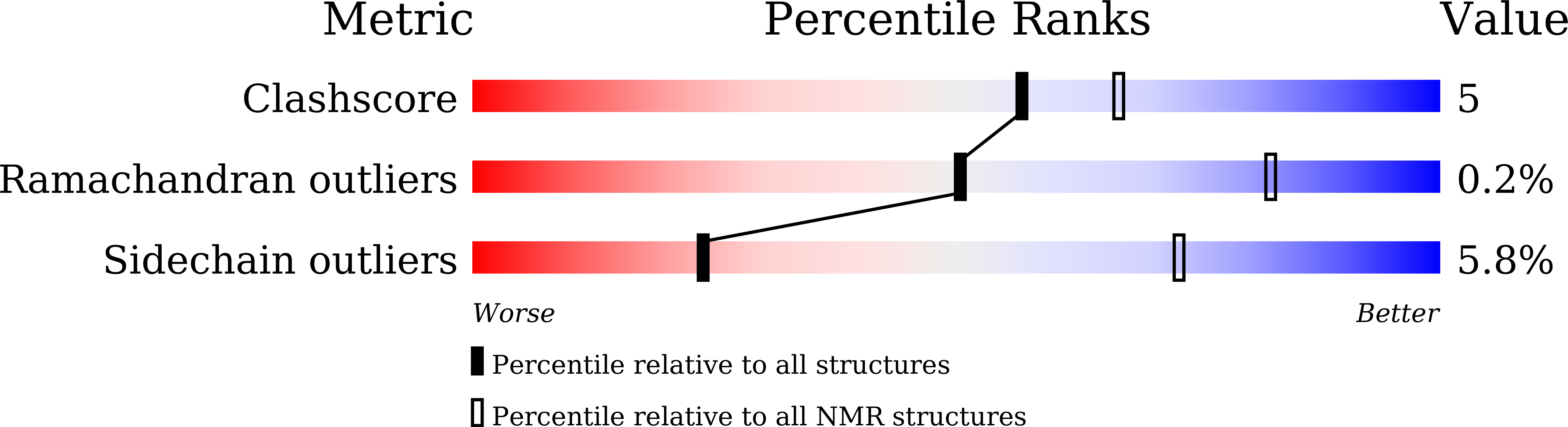Fungal Wound Healing through Instantaneous Protoplasmic Gelation.
Nguyen, T.A., Le, S., Lee, M., Fan, J.S., Yang, D., Yan, J., Jedd, G.(2021) Curr Biol 31: 271
- PubMed: 33186551
- DOI: https://doi.org/10.1016/j.cub.2020.10.016
- Primary Citation of Related Structures:
6LCI - PubMed Abstract:
Multicellular organisms employ fluid transport networks to overcome the limit of diffusion and promote essential long-distance transport. Connectivity and pressurization render these networks especially vulnerable to wounding. To mitigate this risk, animals, plants, and multicellular fungi independently evolved elaborate clotting and plugging mechanisms. In the septate filamentous fungi, membrane-bound organelles plug septal pores in wounded hyphae. By contrast, vegetative hyphae in the early-diverging Mucoromycota are largely aseptate, and how their hyphae respond to wounding is unknown. Here, we show that wounding in the Mucorales leads to explosive protoplasmic discharge that is rapidly terminated by protoplasmic gelation. We identify Mucoromycota-specific Gellin proteins, whose loss of function leads to uncontrolled wound-induced protoplasmic bleeding. Gellins contain ten related β-trefoil Gll domains, each of which possesses unique features that impart distinct gelation-related properties: some readily unfold and form high-order sheet-like structures when subjected to mechanical force from flow, while others possess hydrophobic motifs that enable membrane binding. In cell-free reconstitution, sheet-like structures formed by a partial Gellin incorporate membranous organelles. Together, these data define a mechanistic basis for regulated protoplasmic gelation, and provide new design principles for the development of artificial flow-responsive biomaterials.
Organizational Affiliation:
Temasek Life Sciences Laboratory, National University of Singapore, Singapore 117604; Department of Biological Sciences, National University of Singapore, Singapore 117543.