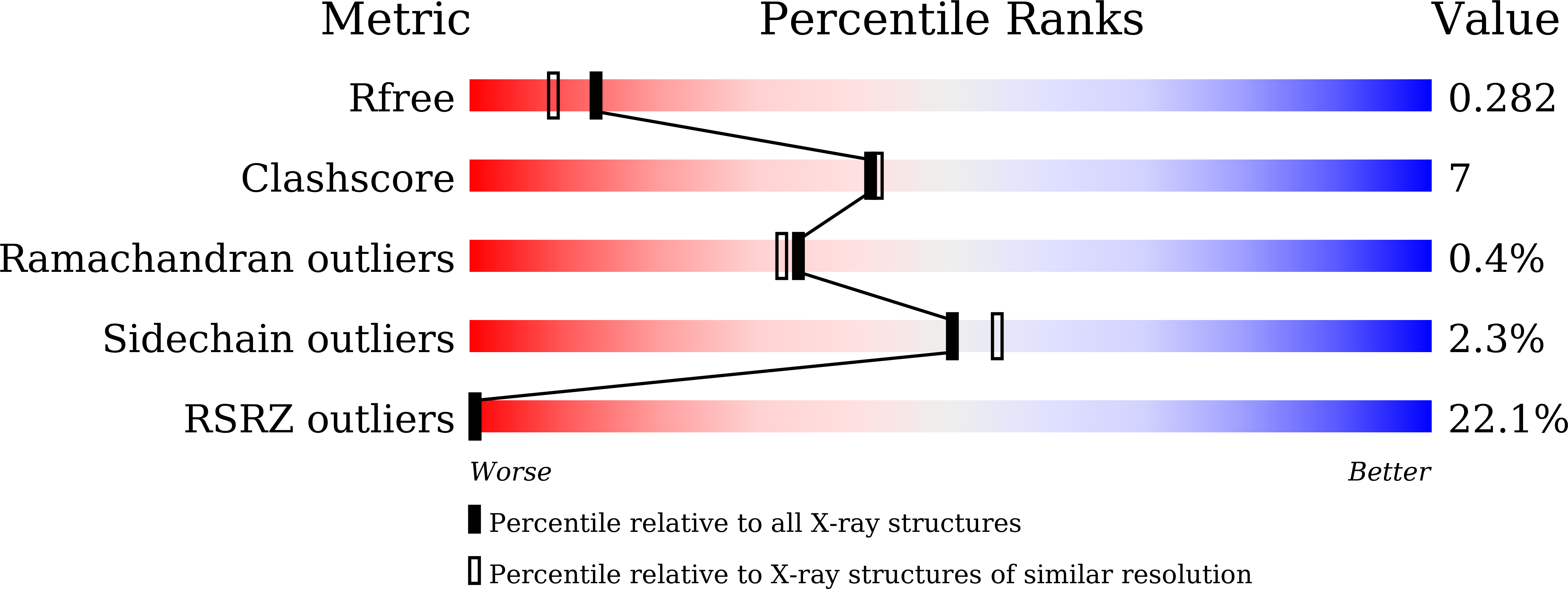
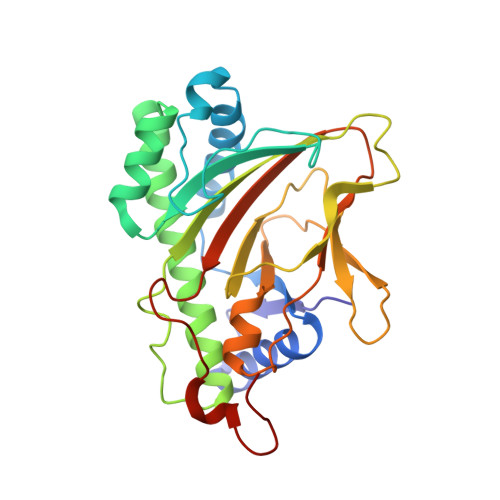
Crystal structure of the indole-3-acetic acid-catabolizing enzyme DAO1 from Arabidopsis thaliana.
Jin, S.H., Lee, H., Shin, Y., Kim, J.H., Rhee, S.(2020) J Struct Biol 212: 107632-107632
- PubMed: 32980521
- DOI: https://doi.org/10.1016/j.jsb.2020.107632
- Primary Citation of Related Structures:
6KWA, 6KWB - PubMed Abstract:
Indole-3-acetic acid (IAA), the major form of the plant hormone auxin, regulates almost every aspect of plant growth and development. Therefore, auxin homeostasis is an essential process in plants. Different metabolic routes are involved in auxin homeostasis, but the catabolic pathway has remained elusive until recent studies identified DIOXYGENASE FOR AUXIN OXIDATION (DAO) from rice and Arabidopsis thaliana. DAO, a member of the 2-oxoglutarate/Fe(II)-dependent oxygenase (2ODO) family, constitutes a major enzyme for IAA catabolism. This enzyme catalyzes, with the cosubstrate 2-oxoglutarate, the conversion of IAA into 2-oxoindole-3-acetic acid, a functionally inactive oxidative product of IAA. Here, we report a crystal structure of the unliganded DAO1 from A. thaliana (AtDAO1) and its complex with 2-oxoglutarate. AtDAO1 is structurally homologous with members of the 2ODO family but exhibits unique features in the prime substrate IAA binding site. We provide structural analyses of a putative binding site for IAA, supporting possible structural determinants for the substrate specificity of AtDAO1 toward IAA.
Organizational Affiliation:
Department of Agricultural Biotechnology, Seoul National University, Seoul, Republic of Korea.