PorA, a conserved C-terminal domain-containing protein, impacts the PorXY-SigP signaling of the type IX secretion system.
Yukitake, H., Shoji, M., Sato, K., Handa, Y., Naito, M., Imada, K., Nakayama, K.(2020) Sci Rep 10: 21109-21109
- PubMed: 33273542
- DOI: https://doi.org/10.1038/s41598-020-77987-y
- Primary Citation of Related Structures:
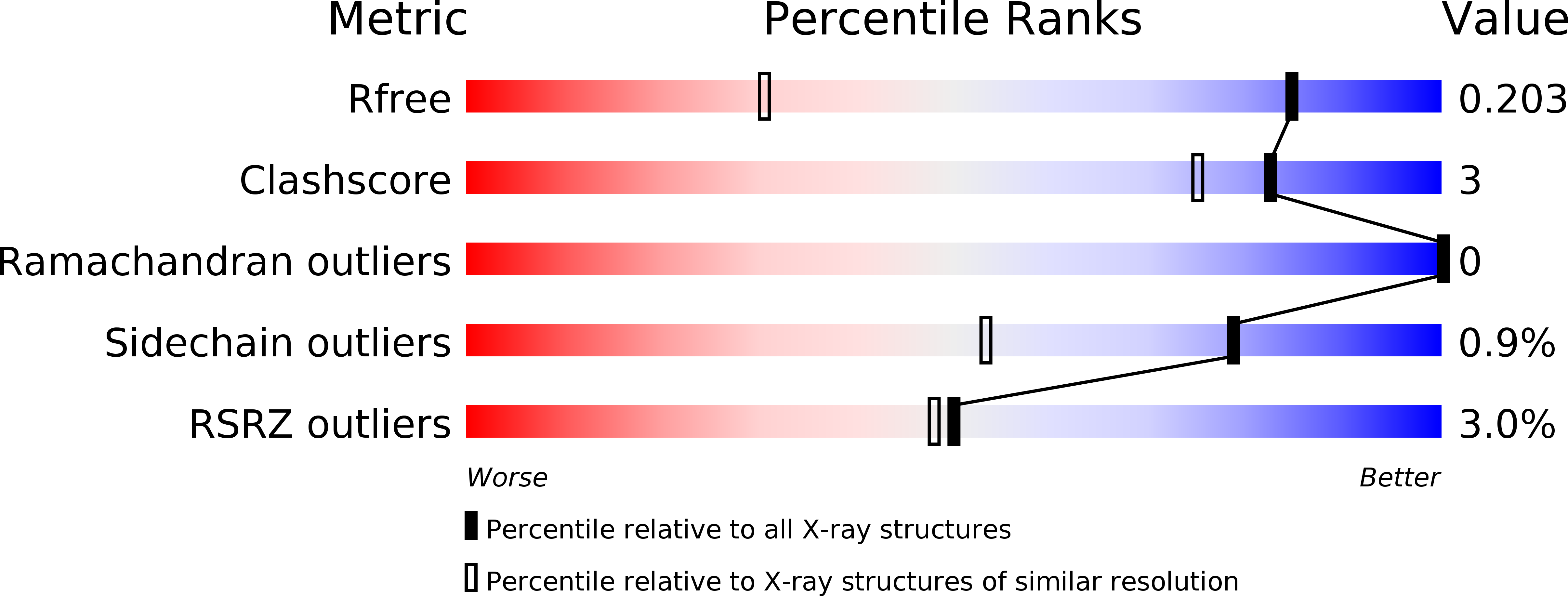
6KJK - PubMed Abstract:
Porphyromonas gingivalis, a periodontal pathogen, translocates many virulence factors including the cysteine proteases referred to as gingipains to the cell surface via the type IX secretion system (T9SS). Expression of the T9SS component proteins is regulated by the tandem signaling of the PorXY two-component system and the ECF sigma factor SigP. However, the details of this regulatory pathway are still unknown. We found that one of the T9SS conserved C-terminal domain-containing proteins, PGN_0123, which we have designated PorA, is involved in regulating expression of genes encoding T9SS structural proteins and that PorA can be translocated onto the cell surface without the T9SS translocation machinery. X-ray crystallography revealed that PorA has a domain similar to the mannose-binding domain of Escherichia coli FimH, the tip protein of Type 1 pilus. Mutations in the cytoplasmic domain of the sensor kinase PorY conferred phenotypic recovery on the ΔporA mutant. The SigP sigma factor, which is activated by the PorXY two-component system, markedly decreased in the ΔporA mutant. These results strongly support a potential role for PorA in relaying a signal from the cell surface to the PorXY-SigP signaling pathway.
Organizational Affiliation:
Department of Microbiology and Oral Infection, Graduate School of Biomedical Sciences, Nagasaki University, Nagasaki, Nagasaki, 852-8588, Japan.