Structural insights into BIC-mediated inactivation of Arabidopsis cryptochrome 2.
Ma, L., Wang, X., Guan, Z., Wang, L., Wang, Y., Zheng, L., Gong, Z., Shen, C., Wang, J., Zhang, D., Liu, Z., Yin, P.(2020) Nat Struct Mol Biol 27: 472-479
- PubMed: 32398826
- DOI: https://doi.org/10.1038/s41594-020-0410-z
- Primary Citation of Related Structures:
6K8I, 6K8K - PubMed Abstract:
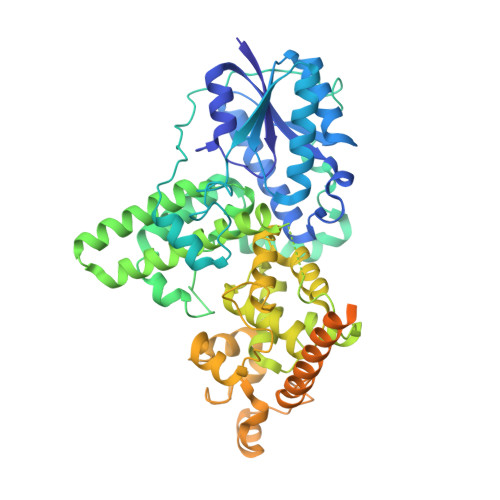
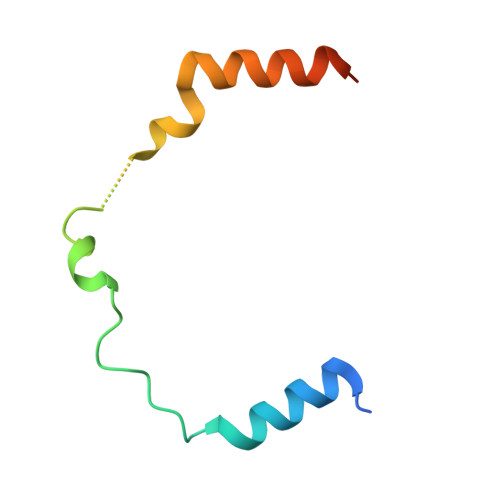
Cryptochromes (CRYs) are blue-light receptors in plants that harbor FAD as a cofactor and regulate various physiological responses. Photoactivated CRYs undergo oligomerization, which increases the binding affinity to downstream signaling partners. Despite decades of research on the activation of CRYs, little is known about how they are inactivated. Binding of blue-light inhibitors of cryptochromes (BICs) to CRY2 suppresses its photoactivation, but the underlying mechanism remains unknown. Here, we report crystal structures of CRY2N (CRY2 PHR domain) and the BIC2-CRY2N complex with resolutions of 2.7 and 2.5 Å, respectively. In the BIC2-CRY2N complex, BIC2 exhibits an extremely extended structure that sinuously winds around CRY2N. In this way, BIC2 not only restrains the transfer of electrons and protons from CRY2 to FAD during photoreduction but also interacts with the CRY2 oligomer to return it to the monomer form. Uncovering the mechanism of CRY2 inactivation lays a solid foundation for the investigation of cryptochrome protein function.
Organizational Affiliation:
National Key Laboratory of Crop Genetic Improvement and National Centre of Plant Gene Research, Huazhong Agricultural University, Wuhan, China.