Structures of two ArsR As(III)-responsive transcriptional repressors: Implications for the mechanism of derepression.
Prabaharan, C., Kandavelu, P., Packianathan, C., Rosen, B.P., Thiyagarajan, S.(2019) J Struct Biol 207: 209-217
- PubMed: 31136796
- DOI: https://doi.org/10.1016/j.jsb.2019.05.009
- Primary Citation of Related Structures:
6J05, 6J0E - PubMed Abstract:
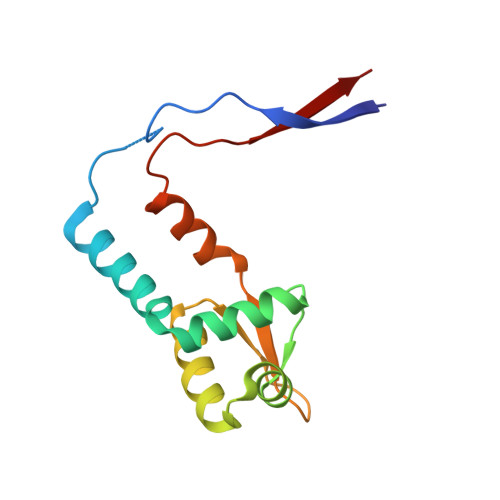
ArsR As(III)-responsive transcriptional repressors, members of the ArsR/SmtB family of metalloregulatory proteins, have been characterized biochemically but, to date, no As(III)-bound structure has been solved. Here we report two crystal structures of ArsR repressors from Acidithiobacillus ferrooxidans (AfArsR) and Corynebacterium glutamicum (CgArsR) in the As(III)-bound form. AfArsR crystallized in P2 1 space group and diffracted up to 1.86 Å. CgArsR crystallized in P2 1 2 1 2 1 and diffracted up to 1.6 Å. AfArsR showed one As(III) bound in one subunit of the homodimer, while the CgArsR structure showed two As(III) bound with S 3 coordination, one in each monomer. Previous studies indicated that in AfArsR As(III) binds to Cys95, Cys96 and Cys102 from the same monomer, while, in CgArsR, to Cys15, Cys16 from one monomer and Cys55 from the other monomer. The dimer interfaces of these structures showed distinct differences from other members of the ArsR/SmtB family of proteins, which potentially renders multiple options for evolving metal(loid) binding sites in this family of proteins. Also, CgArsR presents a new α2-N binding site, not the previously predicted α3-N site. Despite differences in the location of the binding cysteines in the primary sequences of these proteins, the two metal binding sites are almost congruent on their structures, an example of convergent evolution. Analyses of the electrostatic surface of the proteins at the DNA binding domain indicate that there two different modes of derepression in the ArsR/SmtB family of metalloregulatory proteins.
Organizational Affiliation:
Institute of Bioinformatics and Applied Biotechnology, Bengaluru, Karnataka 560100, India. Electronic address: prabaharan@ibab.ac.in.