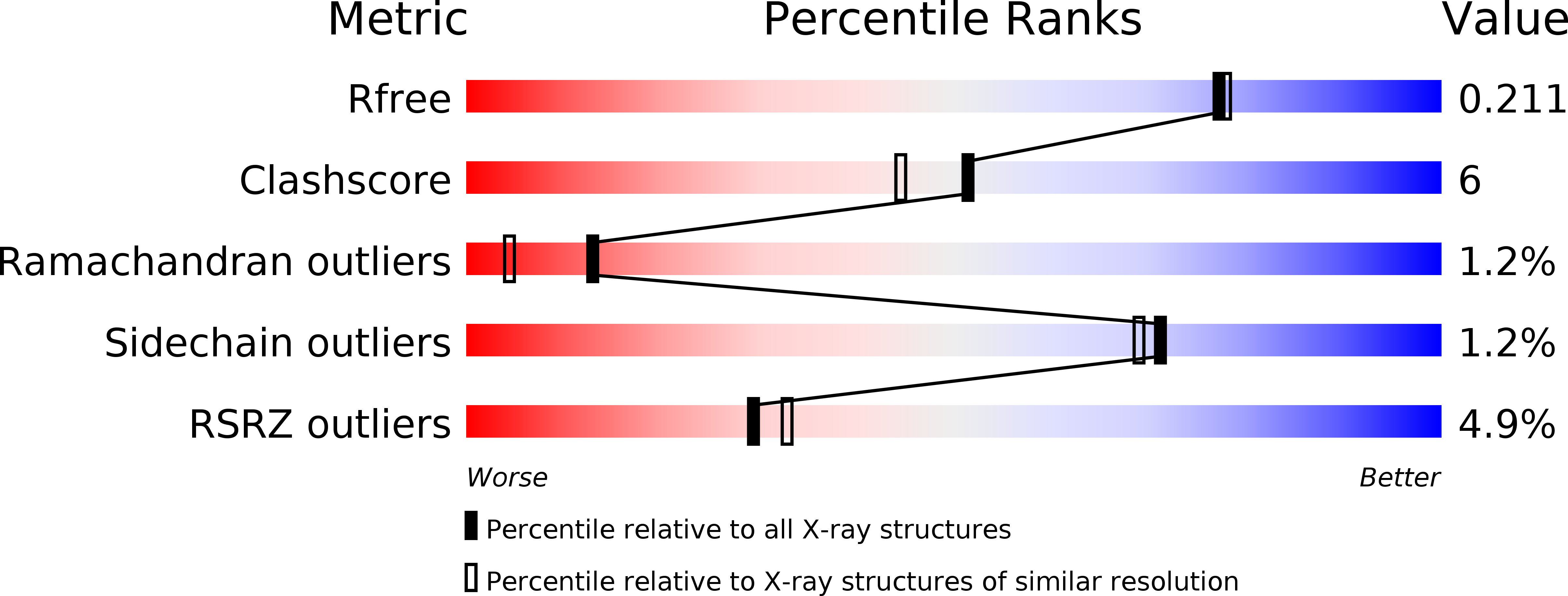
Crystal structure of CagV, the Helicobacter pylori homologue of the T4SS protein VirB8.
Wu, X., Zhao, Y., Sun, L., Jiang, M., Wang, Q., Wang, Q., Yang, W., Wu, Y.(2019) FEBS J 286: 4294-4309
- PubMed: 31230405
- DOI: https://doi.org/10.1111/febs.14971
- Primary Citation of Related Structures:
6IQT - PubMed Abstract:
The VirB/D type IV secretion system (T4SS) plays an essential role in materials transport between host cells and pathogenic Helicobacter pylori and is considered the major pathogenic mediator of H. pylori-associated gastric disease. VirB8, an inner membrane protein that interacts with many other proteins, is a crucial component for secretory function. Here, we present a crystal structure of the periplasmic domain of CagV, the VirB8 counterpart in the H. pylori Cag-T4SS. The structure reveals a fold similar to that of other VirB8 members except for the absence of the α5 helix, a discontinuous β1 strand, a larger angle between the α2 and α3 helices, a more hydrophobic surface groove, but exhibits a different dimer interface. Whether the dimerization occurs in solution was proved by mutagenesis, size-exclusion chromatography and cross-linking assays. Unlike the classical dimerization mode, the interface of the CagV dimer is principally formed by several hydrogen bonds, which indicates instability of dimerization. The structure here demonstrates the difference in dimerization among VirB8 homologues and indicates the considerable compositional and functional diversity of them in T4SS. DATABASE: Coordinates and structure factors have been deposited in the Protein Data Bank under accession codes 6IQT.
Organizational Affiliation:
State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, China.