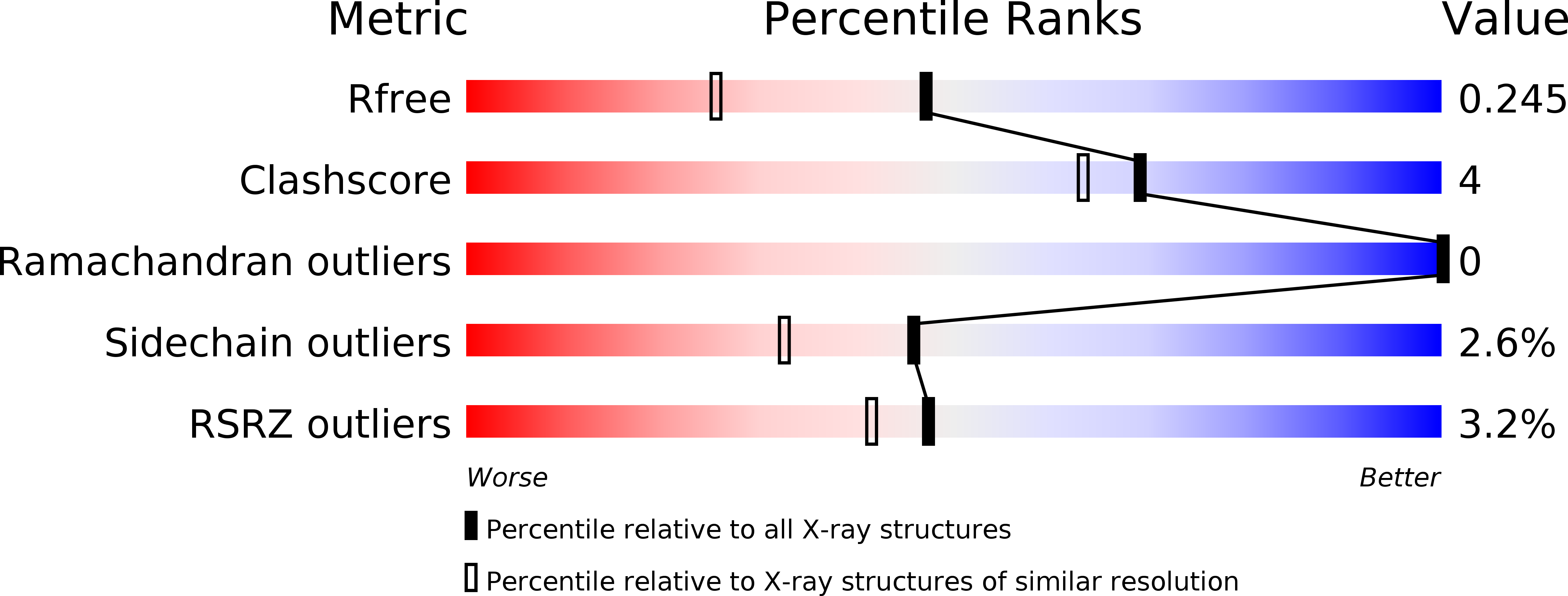
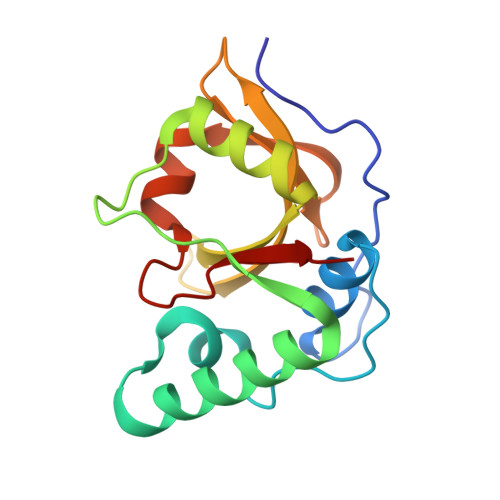
The structure of Erwinia amylovora AvrRpt2 provides insight into protein maturation and induced resistance to fire blight by Malus × robusta 5.
Bartho, J.D., Demitri, N., Bellini, D., Flachowsky, H., Peil, A., Walsh, M.A., Benini, S.(2019) J Struct Biol 206: 233-242
- PubMed: 30928616
- DOI: https://doi.org/10.1016/j.jsb.2019.03.010
- Primary Citation of Related Structures:
6HQZ - PubMed Abstract:
The AvrRpt2 protein of the phytopathogenic bacterium Erwinia amylovora (AvrRpt2 EA ) is a secreted type III effector protein, which is recognised by the FB_MR5 resistance protein of Malus × robusta 5, the only identified resistance protein from a Malus species preventing E. amylovora infection. The crystal structure of the immature catalytic domain of AvrRpt2 EA, a C70 family cysteine protease and type III effector, was determined to a resolution of 1.85 Å. The structure provides insights into the cyclophilin-dependent activation of AvrRpt2, and identifies a cryptic leucine of a non-canonical cyclophilin binding motif. The structure also suggests that residue Cys156, responsible for the gene induced resistance, is not involved in substrate determination, and hints that recognition by FB_MR5 is due to direct interaction.
Organizational Affiliation:
Bioorganic Chemistry and Bio-Crystallography Laboratory (B(2)Cl), Faculty of Science and Technology, Free University of Bolzano, Piazza Università 5, 39100 Bolzano, Italy.