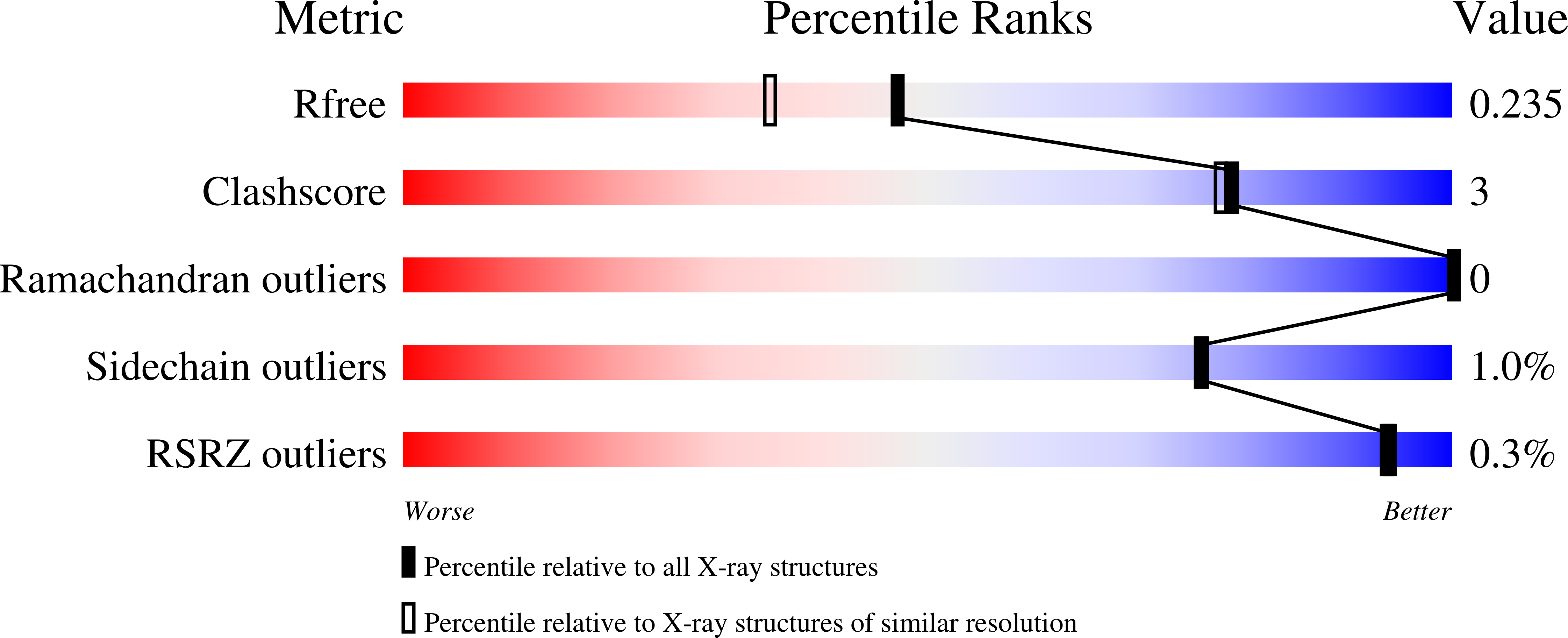
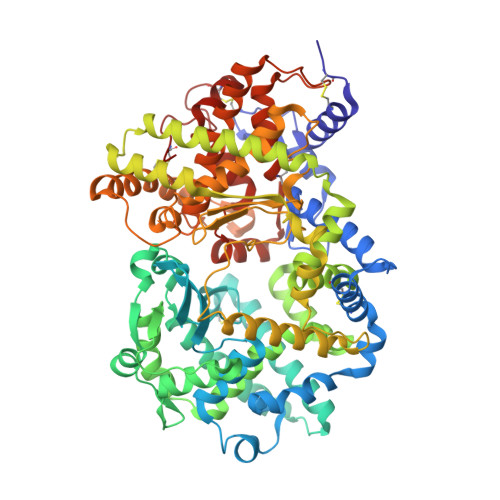
High resolution crystal structure of substrate-free human neprilysin.
Moss, S., Subramanian, V., Acharya, K.R.(2018) J Struct Biol 204: 19-25
- PubMed: 29906506
- DOI: https://doi.org/10.1016/j.jsb.2018.06.004
- Primary Citation of Related Structures:
6GID - PubMed Abstract:
Neprilysin is a transmembrane M13 zinc metalloprotease responsible for the degradation of several biologically active peptides including insulin, enkephalin, substance P, bradykinin, endothelin-1, neurotensin and amyloid-β. The protein has received attention for its role in modulating blood pressure responses with its inhibition producing an antihypertensive response. To date, several inhibitor bound crystal structures of the human neprilysin extracellular domain have been determined, but, a structure free of bound inhibitor or substrate has yet to be reported. Here, we report the first crystal structure free of substrate or inhibitor for the extracellular catalytic domain of human neprilysin at 1.9 Å resolution. This structure will provide a reference point for comparisons to future inhibitor or substrate bound structures. The neprilysin structure also reveals that a closed protein conformation can be adopted in protein crystals absent of bound substrate or inhibitor.
Organizational Affiliation:
Department of Biology and Biochemistry, University of Bath, Claverton Down, Bath BA2 7AY, UK.