Self-Assembling Proteins as High-Performance Substrates for Embryonic Stem Cell Self-Renewal.
Hill, C.J., Fleming, J.R., Mousavinejad, M., Nicholson, R., Tzokov, S.B., Bullough, P.A., Bogomolovas, J., Morgan, M.R., Mayans, O., Murray, P.(2019) Adv Mater 31: e1807521-e1807521
- PubMed: 30866118
- DOI: https://doi.org/10.1002/adma.201807521
- Primary Citation of Related Structures:
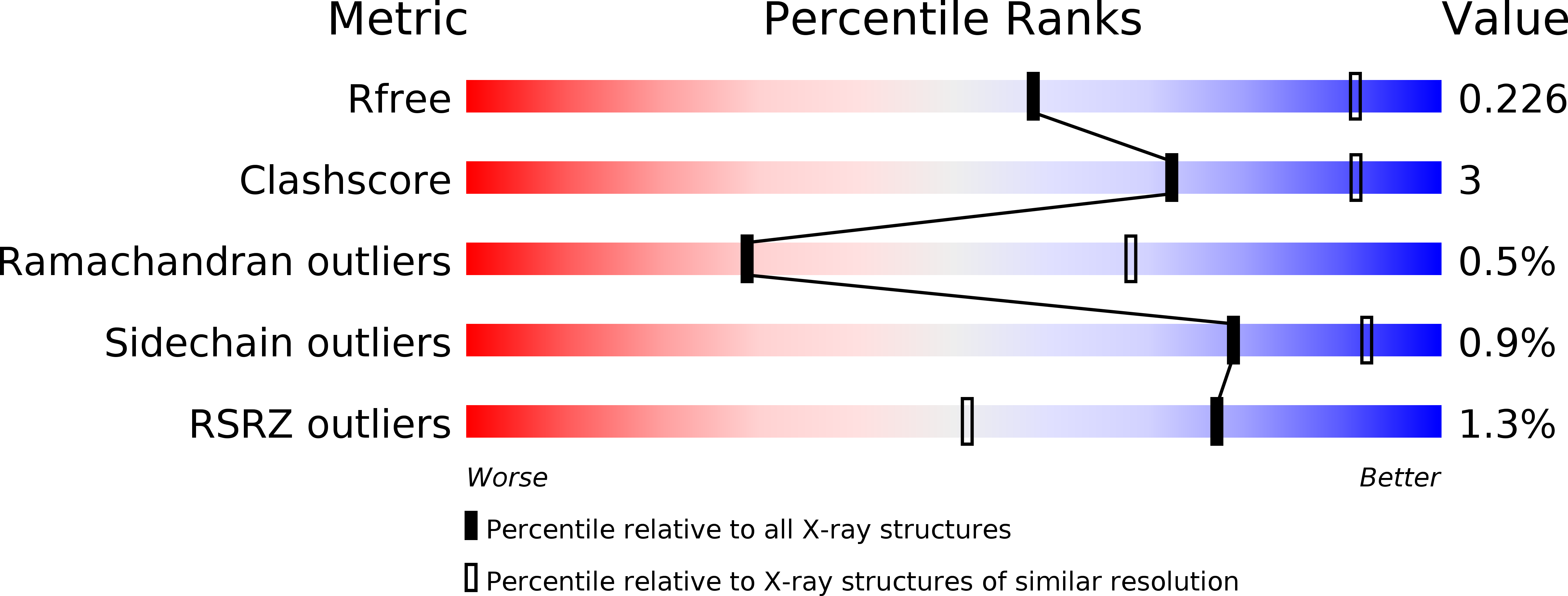
6FWX - PubMed Abstract:
The development of extracellular matrix mimetics that imitate niche stem cell microenvironments and support cell growth for technological applications is intensely pursued. Specifically, mimetics are sought that can enact control over the self-renewal and directed differentiation of human pluripotent stem cells (hPSCs) for clinical use. Despite considerable progress in the field, a major impediment to the clinical translation of hPSCs is the difficulty and high cost of large-scale cell production under xeno-free culture conditions using current matrices. Here, a bioactive, recombinant, protein-based polymer, termed ZT Fn , is presented that closely mimics human plasma fibronectin and serves as an economical, xeno-free, biodegradable, and functionally adaptable cell substrate. The ZT Fn substrate supports with high performance the propagation and long-term self-renewal of human embryonic stem cells while preserving their pluripotency. The ZT Fn polymer can, therefore, be proposed as an efficient and affordable replacement for fibronectin in clinical grade cell culturing. Further, it can be postulated that the ZT polymer has significant engineering potential for further orthogonal functionalization in complex cell applications.
Organizational Affiliation:
Department of Cellular and Molecular Physiology, Institute of Translational Medicine, University of Liverpool, Nuffield Building, Crown Street, Liverpool, L69 3BX, UK.