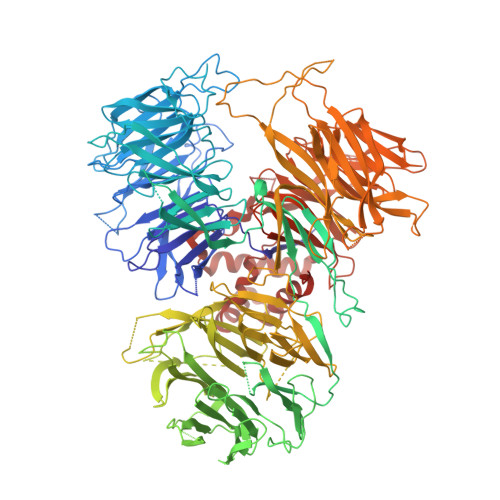
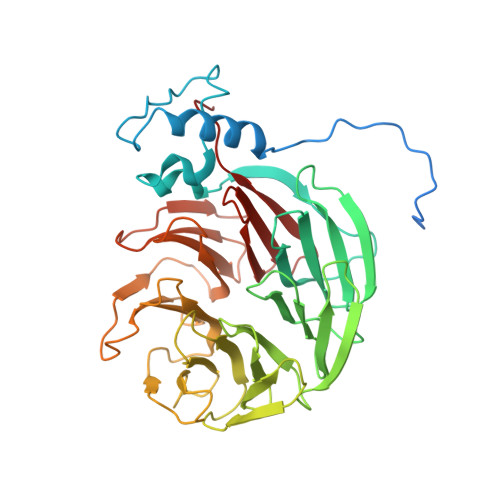
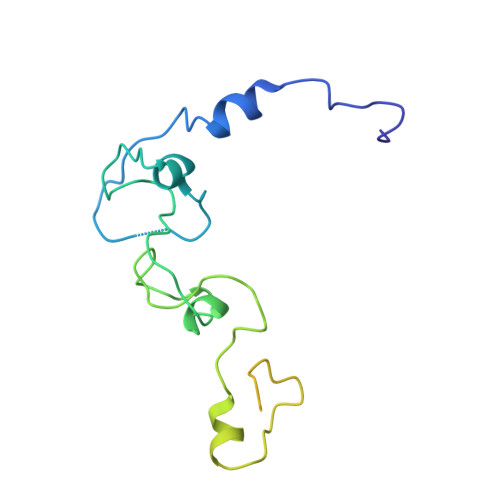
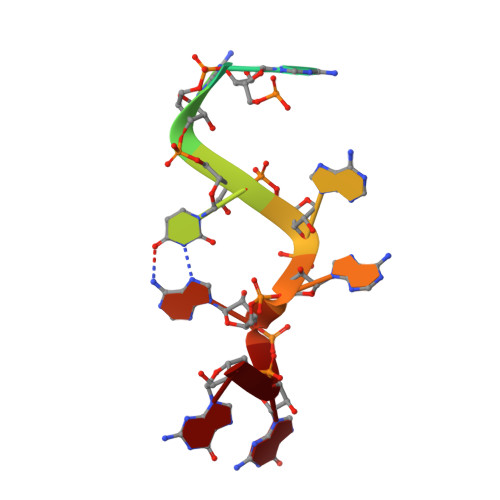
Structural basis of AAUAAA polyadenylation signal recognition by the human CPSF complex.
Clerici, M., Faini, M., Muckenfuss, L.M., Aebersold, R., Jinek, M.(2018) Nat Struct Mol Biol 25: 135-138
- PubMed: 29358758
- DOI: https://doi.org/10.1038/s41594-017-0020-6
- Primary Citation of Related Structures:
6FUW - PubMed Abstract:
Mammalian mRNA biogenesis requires specific recognition of a hexanucleotide AAUAAA motif in the polyadenylation signals (PAS) of precursor mRNA (pre-mRNA) transcripts by the cleavage and polyadenylation specificity factor (CPSF) complex. Here we present a 3.1-Å-resolution cryo-EM structure of a core CPSF module bound to the PAS hexamer motif. The structure reveals the molecular interactions responsible for base-specific recognition, providing a rationale for mechanistic differences between mammalian and yeast 3' polyadenylation.
Organizational Affiliation:
Department of Biochemistry, University of Zurich, Zurich, Switzerland.